Olympus Life Science and Cytosurge have teamed up to present this webinar on how to overcome one of the biggest challenges in gene editing: delivery of reagents into the nucleus.
With traditional delivery methods, such as lipofection or electroporation, the success of CRISPR-Cas mediated gene editing is limited and stressful for the cells. Typically, only one edit can be introduced at once, and its size will be limited. The reagents must pass through the cell membrane, cytoplasm, and nuclear envelope before reaching the nucleus. Traditional delivery methods are not optimized for this long journey, which explains why low transfection efficiency rates are common and often coupled with low viability.
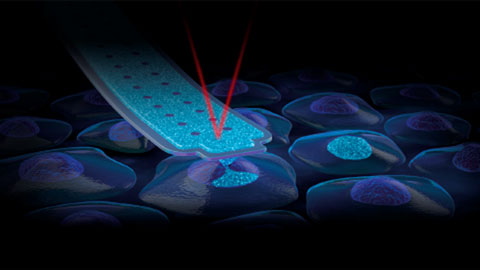
The Cytosurge FluidFM BOT BIO Series can overcome the limitations of traditional transfection methods by directly delivering the desired cargo with a force-sensitive nanosyringe into the nucleus. This method is easy, cargo-independent, and gentle, enabling you to introduce well-defined mixes, such as Cas9, repair templates, and multiple gRNA into the cell nucleus.
Improved efficiency and viability are especially critical when working with rare or hard-to-transfect cells such as induced pluripotent stem cells (iPSCs), neurons, or cardiomyocytes—or when delivering large repair templates.
See these improvements for yourself in this webinar where you’ll learn:
- How the FluidFM BOT BIO Series overcomes established gene editing limitations
- How the nano-injection process works through a live demonstration
FAQWebinar FAQs | FluidFM: a novel approach to CRISPR gene editing by direct intra nuclear deliveryHow long and how often can you use the same probe?While, in theory, you could use the same probe multiple times, it is not recommended due to the high chance of cross-contamination. In principle, the single loading of a probe could be used to inject millions of cells and in practice, thousands of them. Does the probe often get clogged?Although clogging of probes is a familiar difficulty with applications such as micro-injection, it is not something commonly observed with FluidFM. Probe clogging depends on a number of factors, primarily the buffer and the molecule you want to inject; however, it does not appear to be a problem in applications related to CRISPR-Cas9. Additionally, the probe can be coated before the experiment. Not only does this prevent the probe from clogging but it also stops the cells from sticking to the probe after several injections. In fact, we are able to inject several thousands of cells with the same probe without clogging. Can you inject oocytes?Injecting oocytes using FluidFM is an interesting topic, however, as yet, it has not been studied. There is potential to develop a probes and protocols that would make this application possible. Is there any limitation to the dosage or quantity that you can inject?It is possible to adjust the concentration of the liquid loaded into the nano-syringe and subsequently the concentration injected into the cell. Additionally, the pressure can be altered to manage the volume injected. However, large injection volumes can result in cell lysis and are limited to approximately a picolitre. This volume is relevant for all applications. |