Determining Cell Proliferation and Cytotoxicity with Increased Accuracy and Ease Using the CM20 Incubation Monitoring System
Introduction
The cell proliferation/cytotoxicity assay is one of the most frequently used tests in research involving cultured cells. It is an essential preliminary test for examining the concentrations of drugs to be used for treatments and serves as a very important test for determining the efficacy and safety of drugs in various research fields, such as oncology and cell death.
Conventionally, the WST-8 or ATP assay, which use metabolic activity as an index, and the BrdU or thymidine assay, which use the level of DNA synthesis as an index, have been used for the quantitative assessment of the growth characteristics of cells. Although these assays are beneficial due to their simplicity and throughput, they are all indirect assessment methods, and accordingly results may not be correlated with actual number of cells. They are in many cases endpoint assessments, which have sometimes resulted in the failure to capture temporal changes, leading to important findings being overlooked.
Because of these limitations, we consider that the development of a method for direct and temporal assessments of cell proliferation/cytotoxicity is important for research fields that employ cultured cells.
Experimental Outline
To verify the OLYMPUS Provi CM20 incubation monitoring system’s capacity for cell proliferation/toxicity assessment, we conducted the following two assays to compare the CM20 system with a conventional assay (WST-8):
- Cell death assay with anticancer drug (5-FU) in human lung adenocarcinoma A549 cells
- Cell death assay with neurotoxin (6-OHDA) in human neuroblastoma SH-SY5Y cells
Experimental Procedures
Cancer cell death assay with anti-cancer drug (5-FU)
Human lung adenocarcinoma A549 cells were seeded onto a multiwell plate, treated with 5-FU (400 μM) for 72 hours from the time point of 24 hours after seeding, with imaging and counting for cell number every hour by the CM20 system. The 5-FU concentration for treatment was set so cell proliferation and cell death were in equilibrium, with a 5-FU-untreated group set as a control. Conventional methods were used to measure cell viability at 0, 24, 48, and 72 hours after the start of the treatment using the WST-8 assay.
Cell death assay with neurotoxin (6-OHDA)
Human neuroblastoma SH-SY5Y cells were seeded onto a multiwell plate, treated with 6-OHDA (60 μM) for 24 hours from the time point of 24 hours after seeding, with imaging and counting for cell number every hour by the CM20 system. The 6-OHDA concentration for treatment was set to induce rapid cell death, with a 6-OHDA untreated group set as a control. The conventional method measured cell viability at 0, 6, 12, and 24 hours after the start of the treatment using the WST-8 assay.
Results
Cancer cell death assay with an anti-cancer drug (5-FU) and A549 cells
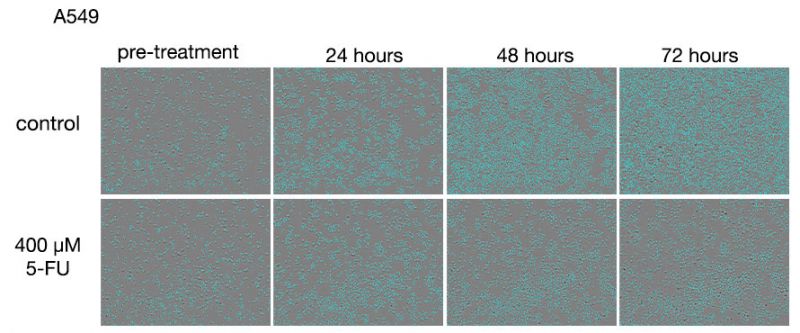
Figure 1. Images of A549 cells after treatments with 5-FU.
Upper row: 5-FU untreated group. Lower row: Group treated with 400 μM 5-FU.
In the 5-FU-untreated group, the number of cells increased with the duration of the culture, and the cells were observed to become 100% confluent near the 72-hour treatment. In the 5-FU-treated group, the number of dead cells increased with the duration of treatment without significant change in cell density after the 24-hour treatment.
|
|
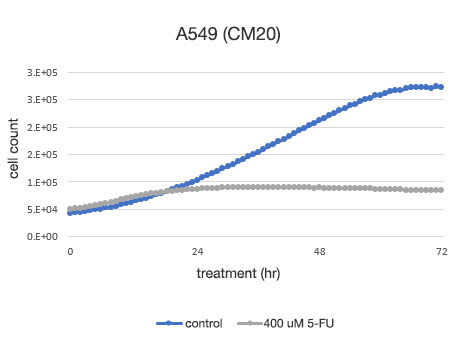
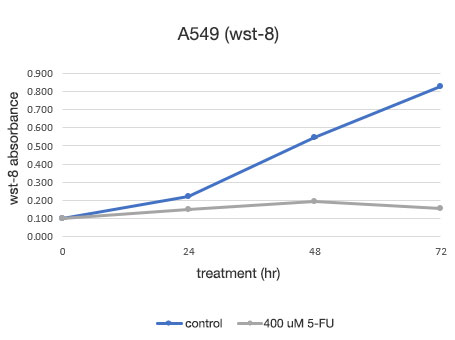
Figure 2. Quantitative determination of the viability of A549 cells.
Left: The number of cells determined by the CM20. Right: The number of cells determined using the conventional assay (WST-8) method.
The analysis with the CM20 system showed that the number of cells increased in a sigmoid curve in the 5-FU-untreated group, while the number of cells did not change significantly after the treatment at 24 hours in the 5-FU-treated group, indicating that cell proliferation and cell death were in equilibrium. These results are consistent with the visual observation of the cells. They are also in agreement with the results obtained by the conventional method (WST-8 assay).
Cancer cell death assay with neurotoxin (6-OHDA) and SH-SY5Y cells
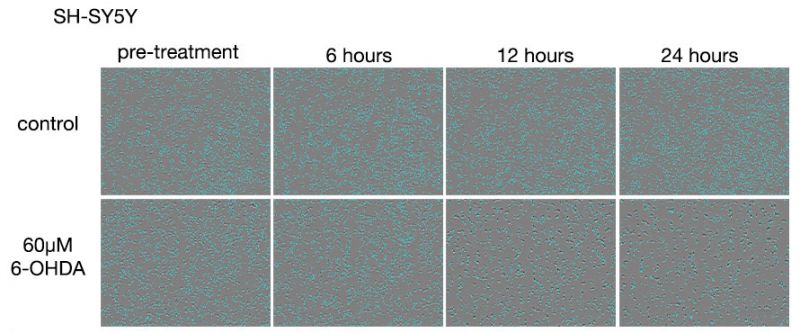
Figure 3. Images of SH-SY5Y cells after 6-OHDA treatments.
Upper row: 6-OHDA untreated group. Lower row: Group treated with 60 μM 6-OHDA.
In the 6-OHDA-untreated group, a slight increase in cell number was observed at the 24-hour point. In the 6-OHDA-treated group, a decrease in cell number was observed at the 6-hour treatment stage, and at the 12-hour treatment, we observed that most of the cells were dead.
|
|
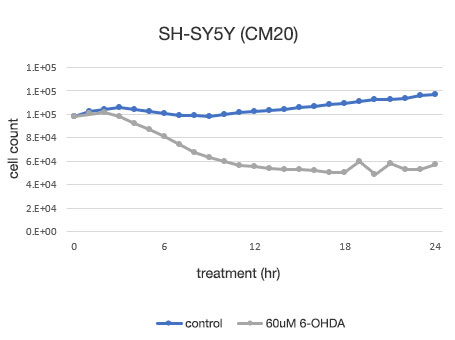
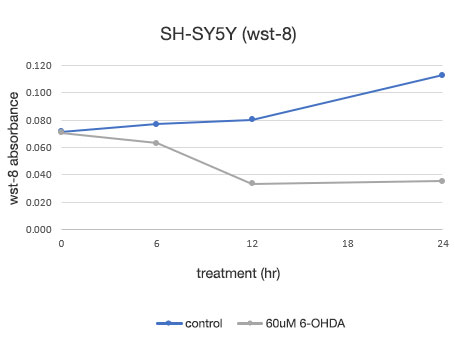
Figure 4. Quantitative determination of the viability of SH-SY5Y cells.
Left: The number of cells determined by the CM20 system. Right: The number of cells determined using the conventional assay (WST-8) method.
The analysis using the CM20 system showed that the number of cells increased slightly in 24 hours in the 6-OHDA-untreated group, while in the 6-OHDA-treated group, the cell number started to decrease at the 3-hour treatment and continued to decrease thereafter. The number of cells did not show significant change after the 12-hour treatment, indicating that almost all the cells died within about 12 hours of treatment. These results are consistent with the visual observation of cells. They are also in agreement with the results using the conventional method (WST-8 assay).
Discussion on the Results Obtained by the CM20 System versus the Conventional Assay
The analysis using the CM20 monitoring system of the action of the anticancer drug in cancer cells and the neurotoxicity of another drug in nervous system cells provided almost the same results as those obtained with image observation and a conventional method (WST-8 assay).
Benefits of the Assessments Using the CM20 Incubation Monitoring System
One of the benefits of using the CM20 system for the assessment is that the short sampling interval enables users to ascertain the details of temporal changes. For example, the test using SH-SY5Y cells shows that almost all the cells died approximately 12 hours after the 6-OHDA treatment. Meanwhile, endpoint assays do not provide such information.
Another benefit of CM20 system is that time-course data can be obtained in only one plate. The conventional method requires the preparation of a plate for each time point of measurement, which increases the amount of work by the number of time points and limits the number plates that can be analyzed. In addition, an increase of measurement points when using a conventional assay method also increases the complexity of the data analysis, whereas the CM20 system enables automatic multipoint observation and automatic output of graphs of the results.
An additional benefit of the CM20 system comes from its automatic image acquisition feature that enables users to review the state of cell proliferation and morphological changes even after the data analysis stage has begun. The CM20 system records the images of cells throughout the analysis so more and potentially crucial information can be gathered and analyzed. Even in cases where the conventional method requires an estimate of the appropriate duration for treatments by analyzing several points followed by retests, the analysis using the CM20 system enables users to determine the appropriate duration for treatments later in a single test and then extract the data within that duration.
Comments by Dr. Yamaguchi
First of all, I was impressed by the accuracy of cell count when using the CM20 incubation monitoring system. The cells recognized by the CM20 system were displayed separately on a monitor, one by one. I confirmed that this assay enables highly reproducible quantitative assessments when the initial parameters are set properly.
Unlike the conventional indirect cell-number measurement method, the CM20 system does not require any labeling. Since labeling can impact cells, avoiding this procedure makes the CM20 system a reliable assay method for the assessment of cell numbers. In addition, the appeal of the CM20 system is that it enables almost automatic acquisition of data for many time points in a single plate, which greatly reduces the amount of work and helps eliminate the risk of overlooking important data.
Depending on the types and concentrations of drugs used in experiments, some variations may be observed in the middle of treatments even in the event that, for example, no difference is observed at the endpoint where cells are all dead or have reached confluence. I feel that the use of the CM20 system may greatly reduce the time and effort required to estimate the duration of treatment in such cases, which could improve the research efficiency and speed.
Acknowledgments
This application note was prepared with the help of the following researcher:
Takahiro Yamaguchi, PhD, Principal Researcher, ACEL, Inc.
Products related to this application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.