Imaging of Drug Response in Drug Efficacy Assessment
Index
- Spheroid drug response imaging (calcium ion fluctuation)
- Chemiluminescence multipoint observation of multiwell plates
- Long-term observation through medium perfusion and automated substrate addition
- Fruit fly embryogenesis observation
1. Spheroid drug response imaging (calcium ion fluctuation)
Organoids and spheroids (cell aggregates) are 3D models that often respond in ways that more closely resemble actual living tissue compared to conventional evaluation using 2D cultured cells. There is an increasing need for organoids and spheroids in drug development research for drug efficacy evaluation applications. Similarly, the interest in luminescence imaging, which involves the use of luminescence proteins (LP) rather than fluorescence proteins (FP) is on the rise due to advantages including reduced phototoxicity and the absence of background fluorescence noise.
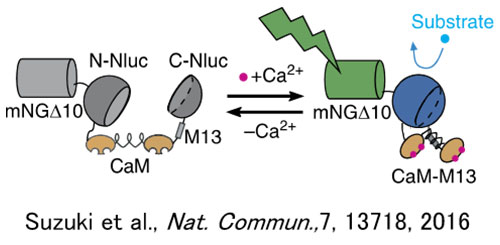
For the investigation of cell receptor GPCR ligands, which are a main target molecular group for pharmaceuticals, calcium ion concentration fluctuations are often used as an indicator. As chemiluminescence (CL) does not require excitation light, unlike fluorescence, autofluorescence of spheroids and other structures is not a concern. Because of this characteristic of CL probes, CL imaging, has the potential to be used for measuring calcium ion concentration fluctuations in spheroids, while maintaining a high signal-to-noise ratio (SNR) and high quantification. Accordingly, GeNL (Ca2+)_520 (41), green enhanced nanolantern (eNL) calcium luminescence sensors, were introduced into spheroids created using cultured cells. After stimulation with histamine, which is a GPCR H1 receptor ligand for spheroids, long-term continuous observation of the cell’s internal calcium ion concentration fluctuations was attempted (21). The result was successful observation of the GeNL(Ca2+)_520 luminous signals with a high SNR for a 50-minute period (Figure 2).
Observation conditions
HEK293T cells: Cultured for one week in a 96-well multiwell U-base plate adeno-associated virus transiently introduced with chemiluminescence calcium ion sensors ((GeNL(Ca2+)_520))
Observation container: Multiwell plate, 96 wells, U-base
Observation medium: DMEM/F-12 (Gibco) +10% FBS
Luminescent substrate:10 µM Furimazine (Promega)
Cell stimulation:2 µM Histamine
Microscope: Luminescence imaging system* based on theIXplore™ Live system
Objective lens: UPLSAPO20X (NA 0.75), camera adaptor: 0.5x
EM-CCD: Andor iXon Ultra 888 (EM-Gain 1000x), exposure time: 20 seconds/photo, pinning: 1 × 1
|
|
Figure 1. Chemiluminescence calcium ion sensor GeNL(Ca2+)_520 actuator operation (left) and an example of a luminescence imaging system based on the IXplore Live system (right) |
Brightfield image | Luminescence image |
Overlay image
|
| Video 1. Luminescence observation of calcium ion fluctuations caused by histamine stimulation (scale bar: 500 µm) |
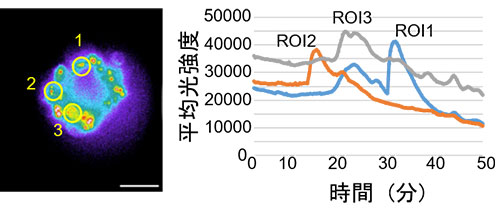
Figure 2. Measurements of calcium ion concentration fluctuations caused by histamine stimulation
2. Chemiluminescence multipoint observation of multiwell plates
When screening new drug candidate molecules from the compound library, it is necessary to perform high-content analysis of cells seeded in a multiwell plate to test for multiple cell phenotypes, such as intracellular ion concentration and changes in cell morphology. In such case, using high-luminosity chemiluminescent proteins is effective because it can quantitatively capture changes in cell morphology and movement with high contrast.
Using chemiluminescence imaging for drug screening and efficacy evaluation, we seeded cultured cells expressing chemiluminescent proteins on a multiwell plate. Repeated automated multipoint scanning was conducted on each well using an motorized stage, and analysis was performed on the acquired imaging data for all wells. This enabled the image acquisition of the entire multiwell plate in just a few minutes. As a result, we were able to successfully observe low-magnification images of the microplate wells as well as high-magnification images of the morphology of the individual cells with high contrast. Images of the cells with fields of view ranging from 1 mm to 100 µm are shown in Figure 3. Our experiment demonstrated that high-throughput and high-content cell analysis can be achieved using a chemiluminescence imaging system incorporating a microscope capable of multipoint scanning.
Observation conditions
HeLa cells: Stable expression of the high-brightness chemiluminescence protein, Yellow-enhanced nanolantern sensor
Observation container: Multiwell plate, 96 wells, flat base
Observation medium: HBSS(-) (Sigma)
Luminescent substrate: 10 µM Furimazine (Promega)
Microscope: Luminescence imaging system* based on the IXplore Live system
Objective lens: UPLFLN10X2PH (NA 0.3), camera adaptor: 0.5x EM-CCD: Andor iXon Ultra 888 (EM-Gain 1000x), exposure time: 1 second/photo, pinning: 2 × 2
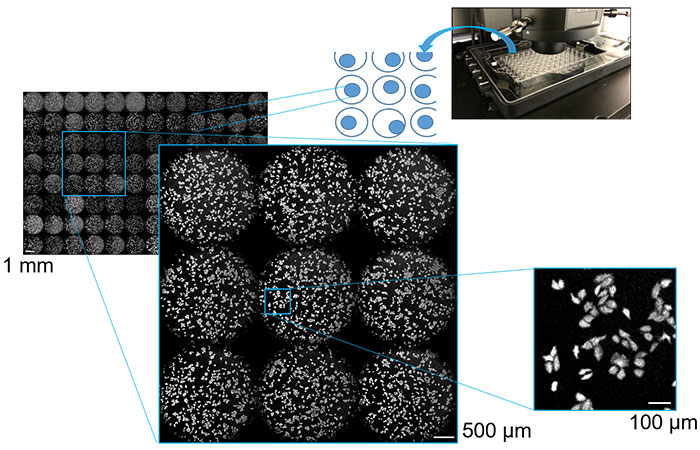
Figure 3. Cultured cells in multiwell plates at 1 mm, 500 µm, and 100 µm captured using multipoint scanning luminescence imaging
3. Long-term observation through medium perfusion and automated substrate addition
For the purpose of drug efficacy evaluation, observation of cells over a long duration is essential for a detailed analysis of the effects. Because of the characteristic short maturation time and half-life of luciferase, they have long been recognized as effective reporter genes to monitor the dynamics of gene expression over time. Also, since excitation light is not required as it is with fluorescence, when using luminescent probes for long-term imaging applications, cell phototoxicity is reduced. However, since this technique requires a luminescent substrate (luciferin), a stable supply of luciferin to the cells is important.
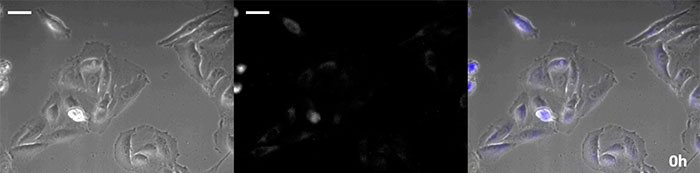
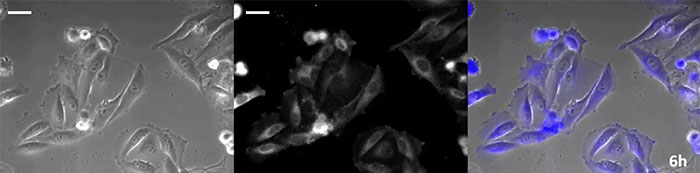
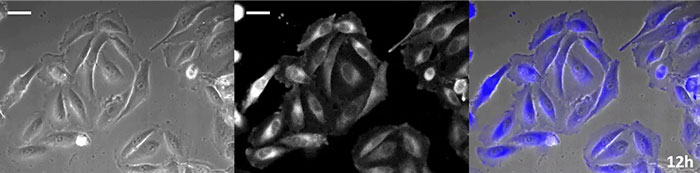
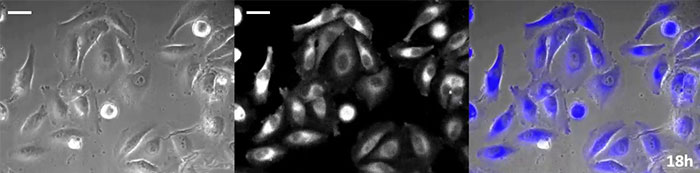
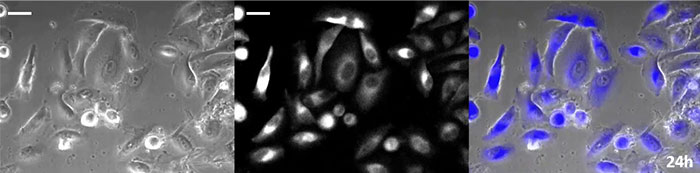
Coelenterazine-type luciferin has especially high brightness, but, since it oxidizes in the cell within in a short time frame, timely addition is essential for long-term observation applications. To resolve this issue, we perfused the cells with high-intensity luminescent protein, while an automated substrate addition device automatically added the coelenterazine so the luminescence could be continually monitored. As a result, we succeeded in monitoring the luminescent image in combination with phase-contrast imaging for more than 24 hours (Figure 4).
Phase-contrast image | Luminescence image |
Overlay image
|
0 hours |
|
6 hours |
|
12 hours |
|
18 hours |
|
24 hours |
|
Observation conditions
HeLa cells†: Stable expression of the high-brightness chemiluminescence protein, Yellow-enhanced nanolantern
Observation container: 35 mm glass-bottom dish
Observation medium: DMEM/F12 (Gibco) +10% FBS
Luminescent substrate: 2.5 mM Coelenterazine-h (FUJIFILM Wako Pure Chemical Corp.), 1.2 µL/7.5 minutes
Perfusion flow rate: 40 µL/minute, medium drainage: approx. 10 mL/hour
Microscope: Luminescence imaging system* based on the IXplore Live system
Objective lens: UPLFLN40XPH (NA 0.75), camera adaptor: 0.5x
EM-CCD: Andor iXon Ultra 888 (EM-Gain 1000x), exposure time:5 minutes/photo, shooting interval: 7.5 minutes, pinning: 1 × 1
4. Fruit fly embryogenesis observation
Drug efficacy evaluation using model organisms is essential for progressing to the next stage of clinical trials. In recent years, flies have gained attention as excellent material for researching human diseases. For example, Vandetanib, a drug which was discovered through the development and usage of a fly medullary thyroid cancer model, has been approved as a treatment drug for humans by the FDA.
Although fluorescent proteins are often used as reporters for monitoring gene expression in live model organisms, a variety of problems need to be considered, including light transmission from embryos and self-fluorescence in response to excitation light. Luminescence imaging, on the other hand, does not require excitation light, which eliminates a majority of these problems. For this experiment, D-Luciferin mixed with yeast paste was fed to third-instar stage larvae, and the changes in engrailed gene expression through the fruit fly embryogenesis process were monitored over time using this compound as a reporter. The results showed that luminescence could be detected deep inside the chrysalis, through the pupal shell, and we succeeded in real-time observation of changes in the expression sites and expression level during the transformation process (Video 2).
Luminescence image | Luminescence image (pseudo color) |
 |
Video 2. Fruit fly embryo luminescence imaging (scale bar: 500 µm)
Observation conditions
Fruit fly: Engrailed gene expression luminescence reporter (luciferase: Pmat)
After administering D-Luciferin mixed with yeast paste to the third instar larva, the pupae were placed on a 24-well plate for observation
Microscope: Luminescence imaging system* based on the IXplore Live System Object lens: UPLFLN4XPH (NA 0.13), camera adaptor: 0.5x
EM-CCD: Andor iXon Ultra 888 (EM-Gain 300x), exposure time: 120 seconds/photo, pinning: 1 × 1
Acknowledgements
These application notes were prepared with the help of the following researchers:
Professor Kenji Nagai and Assistant Professor Mitsuru Hattori
Osaka University Institute of Scientific and Industrial Research, Nagai Laboratory
The fruit fly embryo samples were prepared with the help of the following researchers:
Professor Toshie Kai and Assistant Professor Ritsuko Sugiyama
Graduate School of Frontier Biosciences Osaka University, Reproductive Biology Lab
*The luminescence imaging system used for these experiments was the result of joint development carried out by Professor Kenji Nagai et al. of The Institute of Science and Technology at Osaka University, Tokai Hit Co. Ltd., and Olympus Corporation as part of an advanced measurement and analysis technology/equipment development program and combing existing product components, including the Olympus IXplore Live microscope. Though this particular system is only available in certain regions, Evident Life Sciences does offer similar luminescence solutions to its global customers. Contact your local Evident sales representative to get more details.
Works cited:
Biochem. Biophys. Rep., 23 (2020) 100771
†Note: HeLa cells are one of the most important and well known cell strains for medical research and scientific development. They have contributed to major discoveries in immunology, infectious diseases, and cancer research, and have raised serious questions about ethics in the medical field. Visit http://henriettalacksfoundation.org/ for more information on the life of Henrietta Lacks and her contributions to modern medicine.
Products Related to This Application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.