Extended Time-Lapse Imaging of Cell Motility and Proliferation Using the Olympus FLUOVIEW FV3000 and TruFocus Z-Drift Compensation System
Cell culture-based imaging assays are foundational pillars for investigating a variety of biological phenomena. Using this technique, researchers can observe cellular proliferation and dynamics over time. Because cell populations can exhibit locally diverse protein expression profiles and patterns of motility, imaging assays need to be carefully designed to avoid biases and skewed results created by insufficient sampling. Thus, cell culture-based imaging experiments should be designed to accommodate multiple fields of view across the microplate well or culture device. This application note explores how the FV3000 microscope can facilitate capturing multi-area time-lapse images with complex acquisition parameters for thorough and robust datasets from a single experiment.
Capturing Multiple Fields of View in a Single Experiment
In this experiment, the FV3000 confocal microscope’s multi-area time-lapse (MATL) module was used to continuously monitor the motility and proliferation of human umbilical vein endothelial cell (HUVEC) populations expressing cytosolic green fluorescent protein (GFP) during a span of ~12.5 hours. While designing the experiment, it was important to monitor multiple regions with various magnifications and Z-stack parameters to track individual cell dynamics as well as the dynamics of the population as a whole. Because the MATL module permits independent XYZT parameters to be registered for each position or tiled area, it was easy to program various imaging conditions for multiple regions with differing magnifications and Z-stack parameters over time.
Figure 1. Multi-area time-lapse experiment schematic. For each well in the well plate, multiple positions of individual and tiled areas were programmed with various independent magnification and Z-stack parameters. These registered positions were imaged in a cycle over the course of 12 hours and 25 minutes.
Using Location-Specific Autofocus to Track HUVEC Cell Motility and Proliferation
One of the biggest challenges in imaging multiple areas over time is the tendency for focus to gradually shift because of small fluctuations in the temperature of the microscope room environment. Additionally, the distance between the coverslip surface and the sample tissue can vary over its surface area, making it even more challenging to obtain clear images over multiple fields of view. The TruFocus Z-drift compensation system combats these problems by utilizing a nonphototoxic near-infrared laser to locate the coverslip-sample interface and determine the corresponding focal position. By combining the TruFocus system with the MATL feature, it is simple to register multiple independent XYZT positions with location-specific autofocus.
In this experiment, multiple regions with differing magnifications and Z-stack parameters were cycled over time to track changes in motility and proliferation of the HUVECs, both at the individual cell level as well as at a population-wide level. As shown in the video highlights in Figure 2, combining MATL with TruFocus system enabled repeated imaging of multiple wells while maintaining sharp focus throughout the course of the 12 hour experiment.
Figure 2. XYZT multi-area time-lapse imaging of HUVECs expressing cytosolic GFP. Shown are 4 sample images of different positions with varying acquisition parameters. The TruFocus system was engaged to keep the sample in focus across the various fields of view throughout the course of the 13-hour experiment.
Imaging conditions
Top two panels:
Objective UPLXAPO20X
Microscope: FLUOVIEW FV3000RS laser scanning confocal microscope
Laser: 488nm (GFP, green)
Scanner: Resonant
Z series: 16 steps
Total number of areas scanned: 14
Bottom two panels:
Objective UPLXAPO10X
Microscope: FLUOVIEW FV3000RS laser scanning confocal microscope
Laser: 488nm (GFP, green)
Scanner: Resonant
Z series: 15 steps
Total number of areas scanned: 48
Total time of experiment: 13 hours and 11 minutes.
How the FV3000 Confocal Microscope Facilitated Our Experiment
Maintaining Focus during Multi-Area Time-Lapses with Olympus’ TruFocus Z-Drift Compensation System
The TruFocus Z-drift compensation (ZDC) system is a fully motorized module that combats focal drift to keep the sample in sharp focus during long-term, time-lapse experiments. The TruFocus system boosts application capabilities by supporting a large number of objectives and vessel types, including both glass- and plastic-bottomed dishes. This enables users to generate clear, focused, and continuous time-lapse data under multiple imaging conditions, without concern for Z-drift.
Multi-Area Time-Lapse (MATL) Software Module Enables Independent XYZT ParametersThe multi-area time-lapse (MATL) software module controls the FV3000 microscope’s motorized XY stage, permitting independent XYZT parameters for each registered position or tiled area. The MATL module provides robust and accurate time-lapse data through complete hardware integration and precise timing provided by the FV3000 inverted microscope, making the system well suited for imaging the numerous commercially available cover-glass bottom cultureware or even custom-made devices and microplates. | 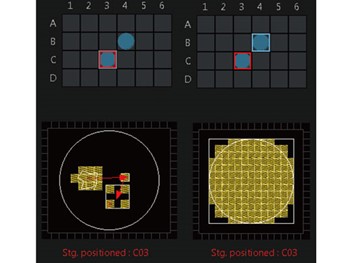 |
|---|
 | Comment by Dr. James HoyingWe work with a variety of 3D vascularized tissue models arrayed across many wells of multi-well plates. The TruFocus system and MATL features of the FV3000 microscope have been invaluable as we often need to make measurements of vascularity and vessel morphology in dozens of tissues at a time using high-resolution, confocal images of the 3D vasculatures. |
|---|
Acknowledgments
This application note was prepared with the help of the following researcher:
Dr. James Hoying, Chief Scientist, Advanced Solutions Life Sciences
Products related to this application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.