Using Intravital Multiphoton Microscopy to Visualize Dynamic Interactions between Neutrophils and Monocytes/Macrophages in an Influenza-Infected Mouse Airway
The innate immune system in the body is the first line of defense against viral infection. One of its major functions is the recruitment of several types of white blood cells, or leukocytes, to the sites of infection (for example, the airway). All these leukocytes, including neutrophils and monocytes/macrophages, act in coordination to build up the body’s defense against the virus. Gaining a deeper understanding of the mechanisms of this innate immune process will help researchers develop and improve the effectiveness of vaccines and therapies.
In this application note, Dr. Minsoo Kim and his colleagues examined the dynamics of neutrophils and monocytes/macrophages in vivo in an influenza-infected mouse trachea using intravital multiphoton microscopy. 3D observation and image analysis revealed previously unrecognized motility patterns of interactions between neutrophils and monocytes/macrophages.
3D reconstruction of images shows neutrophil migration in the influenza-infected mouse trachea. Red, neutrophil; white, blood vessel. Scale bar, 50 μm. tdTomato fluorescence was excited by 975 nm and detected in red channel (575–630 nm). Alexa Fluor 647 fluorescence was excited by 1200 nm and detected in the far-red channel (645–685 nm). (Data adapted from Reference [1])
Neutrophil Migration in the Influenza-Infected Mouse Trachea
To visualize neutrophils in the influenza-infected mouse trachea, Ly6G-Cre/ROSA-tdTomato mice were infected with influenza virus. Within 3–6 days after infection, there was a massive transient infiltration of neutrophils and monocytes into the trachea. To label the blood vessels, Alexa Fluor 647-conjugated CD31 antibody was I.V. injected to the mice before imaging. Intravital multiphoton microscopy was performed on the surgically cannulated trachea of anesthetized mice. As shown in Video 1, both tdTomato+ neutrophils (red) and blood vessels (white) were visualized, showing neutrophil migration around neighboring blood vessels in the influenza-infected mouse trachea.
Motility Patterns of Interactions between Neutrophils and Monocytes/Macrophages
To visualize both neutrophils and monocytes/macrophages in the influenza-infected mouse trachea, Ly6G-Cre/ROSA-tdTomato/Csf1r-EGFP mice were infected with influenza virus. Neutrophils expressed both GFP and tdTomato (showing red/orange fluorescence) while monocytes/macrophages expressed GFP only (showing green fluorescence). As shown in Video 2, GFP+/tdTomato+ neutrophils (red/orange), GFP+ monocytes/macrophages (green), and blood vessels (white) were visualized in the influenza-infected mouse trachea. On the fifth day after infection, most monocytes/macrophages became non-motile, while most neutrophils remained highly motile and constantly moved in close proximity to the surrounding monocytes/macrophages.
Migration and interactions of neutrophil and monocytes/macrophages in the influenza-infected mouse trachea. Red/orange, neutrophil; green, monocytes/macrophage; white, blood vessel. Scale bar, 50 μm. Both GFP and tdTomato fluorescence were excited by 975 nm and then detected in the green channel (495–540 nm) and red channel (575–630 nm), respectively. Alexa Fluor 647 fluorescence was excited by 1200 nm and detected in the far-red channel (645–685 nm). (Data adapted from Reference [1])
A closer look at higher magnification revealed more details in the interaction pattern between neutrophils and monocytes/macrophages. Video 3 shows the 3D reconstruction of neutrophil migration among the monocytes/macrophages in the mouse trachea during the sixth day following infection. This video shows that highly motile neutrophils (red) actively encountered and moved into nonmigratory surrounding monocytes/macrophages (green). Frequent encounters led to the engulfment of neutrophils by monocytes/macrophages, indicating the mechanism of removing the dying neutrophils from the infected trachea.
3D reconstruction of images shows detailed interactions between neutrophil and monocytes/macrophages in the influenza-infected mouse trachea (day 6 post-infection). Red, neutrophil; green, monocytes/macrophage. Both GFP and tdTomato fluorescence were excited by 975 nm and then detected in the green channel (495–540 nm) and red channel (575–630 nm), respectively. (Data adapted from Reference [1])
How the FVMPE-RS Multiphoton Microscope Facilitated Our Experiment
FVMPE-RS Twin-Laser System for Simultaneous Multiwavelength Multiphoton Imaging
Gantry Frame Microscope System for In Vivo Mouse Imaging
Multiphoton Excitation Dedicated Objective XLPLN25XWMP2
| Multiphoton excitation dedicated objectives are optimized for multiphoton imaging deep in tissue. The optical coating offers good transmission from 400 nm to 1600 nm to ensure both efficient near-infrared excitation and visible fluorescence collection. Olympus’ water immersion XLPLN25XWMP2 objective has a high numerical aperture (NA 1.05), long working distance (2.0 mm), and wide field of view (FN 18 mm), which provide excellent performance for in vivo/intravital multiphoton imaging. | 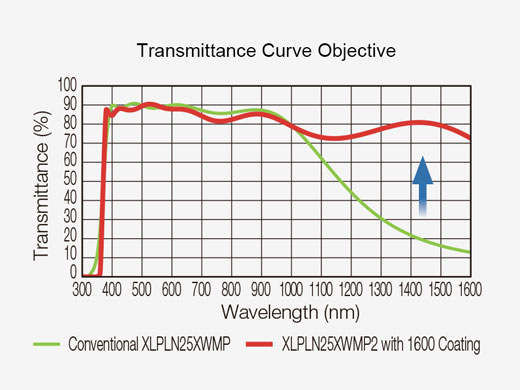 |
|---|
Biography of Dr. Minsoo Kim
Acknowledgments
This application note was prepared with the collaboration of Drs. Minsoo Kim and Kihong Lim at the Department of Microbiology and Immunology, David H. Smith Center for Vaccine Biology and Immunology, University of Rochester, NY, USA.
Reference
Products related to this application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.