Observation of Neural Structures between the Cortex and Thalamus in the Marmoset Brain Using FLUOVIEW FV3000
The prefrontal cortex (PFC) is disproportionately enlarged in humans and is responsible for advanced cognitive and executive functions. Its malfunction can cause psychiatric disorders such as schizophrenia and Alzheimer’s disease. The PFC in mice has been actively studied, but it lacks a region corresponding to the frontal granule cortex, suggesting a large structural difference with that of primates. Research in primate models is, therefore, important to link the mouse and human studies. Our research group is using marmosets, a small monkey native to South America, as the model primate.
In this experiment, we investigated the interactions between the PFC and the thalamic reticular nucleus (TRN), which is a group of inhibitory neurons surrounding the thalamus. The TRN acts like a gate controlling the transmission of information from the cerebral cortex toward the thalamus. We investigated the detailed morphology of the axon fibers in the TRN, which travel from the PFC to the thalamus.
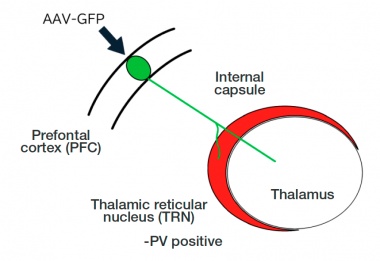
Figure 1. Diagram showing how the neural axons enter the thalamus from the prefrontal cortex through the thalamic reticular nucleus. The thalamic reticular nucleus acts as a gate to the thalamus.
Macro-to-Micro Imaging Using A Simple Workflow
Axon fibers originating from the PFC pass through a corridor called the internal capsule as a thick bundle. This axon bundle enters the thalamus through the anterior part of the TRN, where it shows complex morphologies by splitting and reorienting. To accurately identify the TRN, we used PV (parvalbumin) as the marker (Fig. 1).
The FLUOVIEW FV3000 confocal laser scanning microscope’s macro-to-micro function seamlessly connects macro and micro views for confocal imaging. We used this function to obtain a low-magnification overview image of the PFC axon fibers passing by the PV-positive cells and then switched to high-magnification to observe the branching of fine axon fibers and bouton-like nerve endings.
For the high-magnification observation, a silicone oil immersion objective was used to observe the deeper part of the sample in detail at high resolution. At low magnification, we could just observe the thick axon fibers passing through the TRN. With a 40X silicone oil-immersion objective, we could observe the passing fibers to be finely branched and decorated with innumerable bouton-like granular structures (Fig. 2).
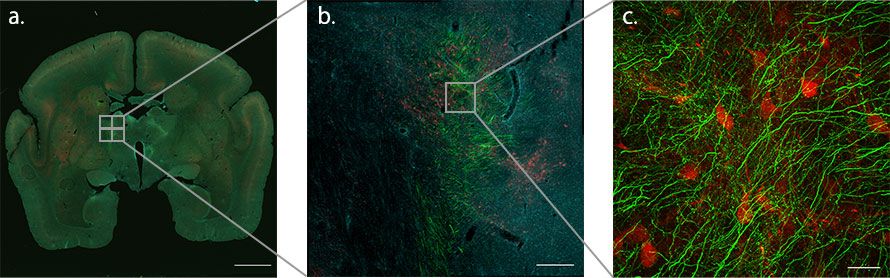
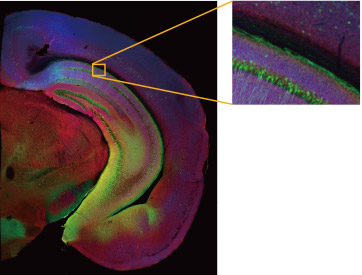
Figure 2. Using the macro-to-micro function to map where the axon fibers meet the TRN on the way to the thalamus from the PFC of the marmoset brain. Since TRN neurons are composed of PV-positive inhibitory neurons, they can be identified by the PV antibodies (red). Green indicates axon terminals from the cerebral cortex and cyan indicates nuclei.
Imaging Conditions
Microscope: FLUOVIEW™ FV3000 confocal laser scanning microscope
Laser: 405 nm (DAPI, cyan), 488 nm (GFP, green), 561 nm (parvalbumin, red)
a. Objective: PLAPON1.25X, Stitching: 3 × 3, scale bar: 3000 μm
b. Objective: UPLXAPO10X, Stitching: 2 × 2, scale bar: 300 μm
c. Objective: UPLSAPO40XS, Stitching: 2 × 2, 73 slices Scale bar: 30 μm (only green and red are displayed)
High-Resolution, Three-Dimensional Observation of the Fine Structure of Axon Fibers
A Z-stack image was then taken using a 100X silicone oil immersion objective for three-dimensional reconstruction (Fig. 3). We were able to observe the detailed three-dimensional structures of the bouton-like granules that surround the TRN neurons.
Figure 3. High-magnification 3D observation of the axon fibers in the TRN on the way to the thalamus from the marmoset PFC | Imaging Conditions |
Comment from Dr. Watakabe
In this experiment, we needed to switch between a low-magnification objective and a high-magnification objective. The macro-to-micro mapping function of the FV3000 microscope enabled this transition smoothly and allowed us to surf through the overall image of the brain, while capturing the fine structure at high magnification. The use of a silicone oil immersion objective helped us observe the fine morphology of the bouton-like nerve endings.
Acknowledgements:Dr. Akiya Watakabe Laboratory for Molecular Analysis of Higher Brain Function, RIKEN Center for Brain Science |
|
Research Background
This research was carried out as a part of the “Brain Mapping by Integrated Neurotechnologies for Disease Studies” (Brain/MINDS) project. This project aims to deepen our understanding of human psychiatric and neurological disorders and eventually overcome them by examining the neural circuits of the primate model. Dr. Watakabe belongs to a research group that is in charge of creating marmoset brain structure maps, focusing on the connections of the prefrontal cortex (PFC) in particular.
Related papers:
Okano, H., Sasaki, E., Yamamori, T., Iriki, A., Shimogori, T., Yamaguchi, Y., Kasai, K., Miyawaki, A. “Brain/MINDS:.” Neuron., 2016, Nov. 2;92(3):582-590. doi:10.1016/j.neuron.2016.10.018.
How the FV3000 Confocal Laser Scanning Microscope Facilitated Our Experiment
Macro-to-Micro Observation for Neural Structure MappingThe FV3000 confocal laser scanning microscope’s macro-to-micro workflow made it possible to capture the structure of the entire tissue and to observe the micro-structure of cells using a simple workflow. |
|
Silicone Oil Immersion Objectives: Enabling Bright Images in Thick Tissue Samples
Olympus’ range of high-performance silicone oil immersion objectives offers high-resolution, deep-tissue observation of transparent specimens. Since the refractive index of silicone oil (about ne 1.40) is close to the refractive index of living tissue (about ne 1.38), these objectives help prevent the spherical aberration caused by refractive index variation, enabling 3D acquisition of tissue structures with high definition.
Related Videos |
Products related to this application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.