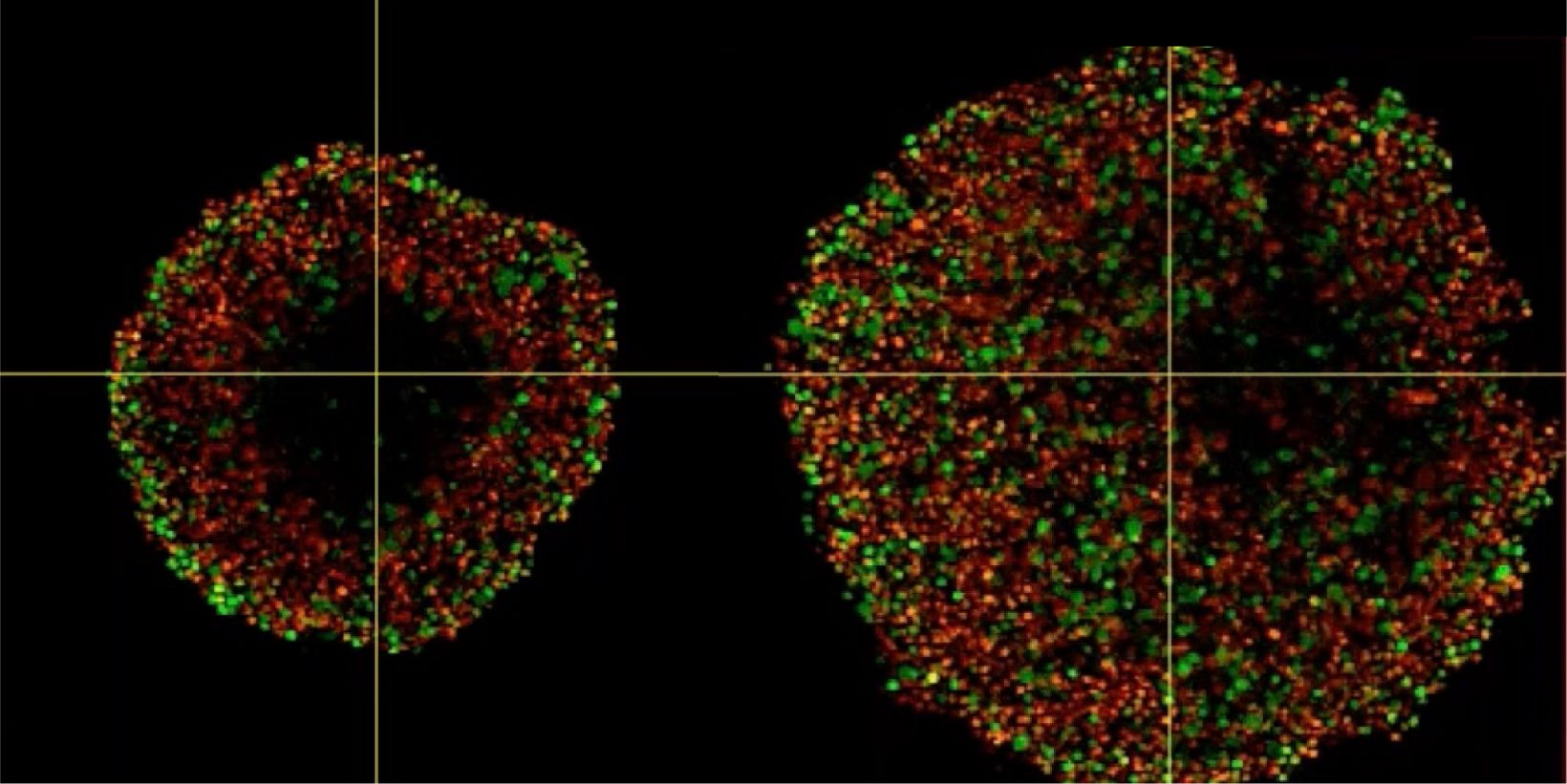
Phenotypic tumour heterogeneity arising due to differentially cycling cell populations has been implicated in increased therapy resistance. This phenomenon cannot be assessed in adherent cell culture, where microenvironmental conditions are homogeneous. Thus, we utilise melanoma spheroids to model the 3D tumour microenvironment including the extracellular matrix (ECM) and study spheroid structure, necrotic region, individual cell arrangement within and gene expression patterns. We achieve this by exploiting the fluorescence ubiquitination cell cycle indicator (FUCCI) system to monitor cell cycle stages as a surrogate marker for phenotypic tumour heterogeneity, tissue clearing and confocal microscopy using FV3000.
Investigating Spheroid Architecture Using the FV3000 Confocal MicroscopePhenotypic tumour heterogeneity arising due to differentially cycling cell populations has been implicated in increased therapy resistance. This phenomenon cannot be assessed in adherent cell culture, where microenvironmental conditions are homogeneous. Thus, we utilise melanoma spheroids to model the 3D tumour microenvironment including the extracellular matrix (ECM) and study spheroid structure, necrotic region, individual cell arrangement within and gene expression patterns. We achieve this by exploiting the fluorescence ubiquitination cell cycle indicator (FUCCI) system to monitor cell cycle stages as a surrogate marker for phenotypic tumour heterogeneity, tissue clearing and confocal microscopy using FV3000. | |
Related ProductsConfocal Laser Scanning Microscope FV3000Optimized for high sensitivity and low phototoxicity, the FLUOVIEW™ FV3000 system provides the performance and flexibility to meet the challenges of live cell and deep tissue observation. The TruSpectral™ detector’s sensitivity to the 400 nm–800 nm wavelength range enables the detection of even dim fluorophores. Adjusting the detection wavelength at the resolution of 1 nm, enables the efficient capture of high-resolution 3D images. Advanced imaging mode options include macro-to-micro, super resolution, and quantitative data analysis.
| |
Investigating Spheroid Architecture Using the FV3000 Confocal Microscope
Related Videos
Related Products
FV3000
Optimized for high sensitivity and low phototoxicity, the FLUOVIEW™ FV3000 system provides the performance and flexibility to meet the challenges of live cell and deep tissue observation. The TruSpectral™ detector’s sensitivity to the 400 nm–800 nm wavelength range enables the detection of even dim fluorophores. Adjusting the detection wavelength at the resolution of 1 nm, enables the efficient capture of high-resolution 3D images. Advanced imaging mode options include macro-to-micro, super resolution, and quantitative data analysis.
- Available with either galvanometer (FV3000) or hybrid galvanometer/resonant (FV3000RS) scanner configurations
- TruFocus™ Z-drift compensation system maintains focus during live cell imaging despite changes in temperature or added reagents
- Inverted and upright frame options to suit a variety of applications and sample types
- FV3000 Red near-infrared (NIR) solution extends the wavelength detection capabilities to the NIR region of up to 890 nm, enabling up to 6-channel simultaneous detection for multiplexing