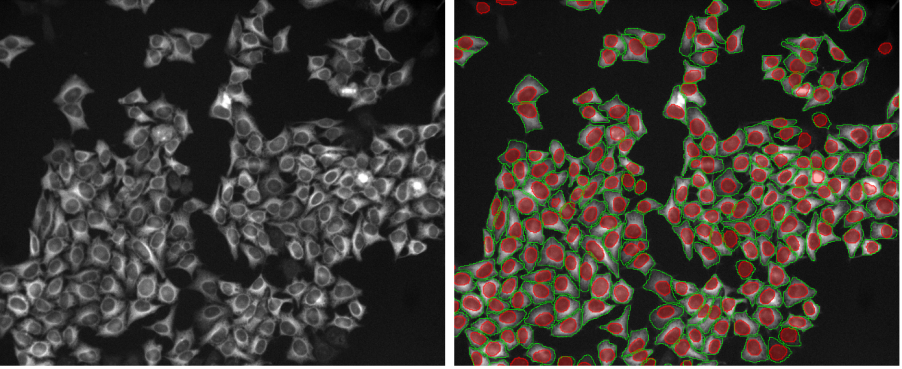
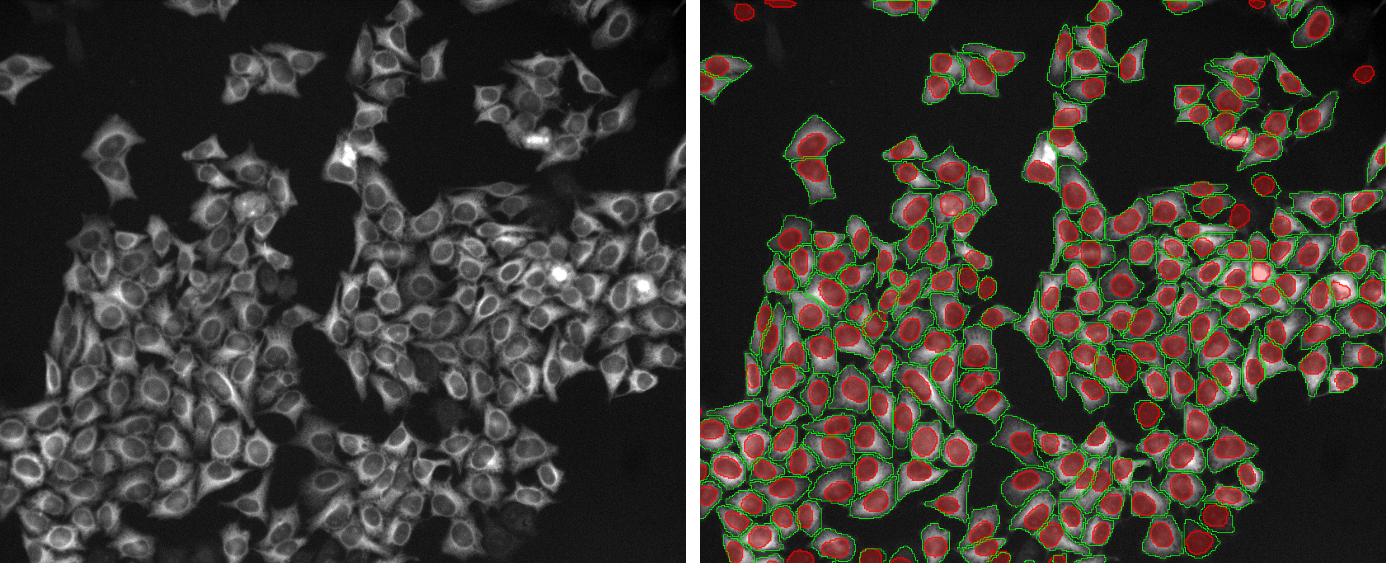
TruAI™ deep-learning technology is a breakthrough image analysis approach because it enables researchers to tackle applications that were impossible or challenging, such as reliable label-free analysis or tracking of living cells at very low intensities over several days.
For these applications, the analysis is performed through semantic segmentation—the ability to identify if a pixel belongs to the background or foreground of a given class. After semantic segmentation, post-processing steps such as watershed are applied to perform the final object segmentation (typically nuclei or whole cells).
Recently, TruAI deep-learning technology was improved with the introduction of instance segmentation. This capability merges the semantic segmentation and the subsequent splitting of objects into one step. Instance segmentation improves workflows by enabling you to tackle difficult applications in one step, such as segmentation of cells in cell colonies that require post-processing splitting steps.
TruAI deep-learning technology enables users to train with their own data from the start, so the generated TruAI deep neural network (DNN) models have extremely good performance. Researchers can develop libraries of DNNs for different applications and even share them with collaborators.
Yet, this implies that the deep-learning workflow always consists of three steps:
- Labeling with your own data
- Training
- Inference
Despite our user-friendly software, the labeling step is the most time-consuming task—especially for challenging applications where many objects need to be labeled to achieve good performance.
Simplified Segmentation of Cells and Nuclei Using Pretrained Deep-Learning Models
To improve this workflow, TruAI deep-learning technology now includes some pretrained models for nuclei and cells, which are the most common objects to be segmented in bioimage analysis. As a result, users can save the time spent labeling and training (Figure 1).
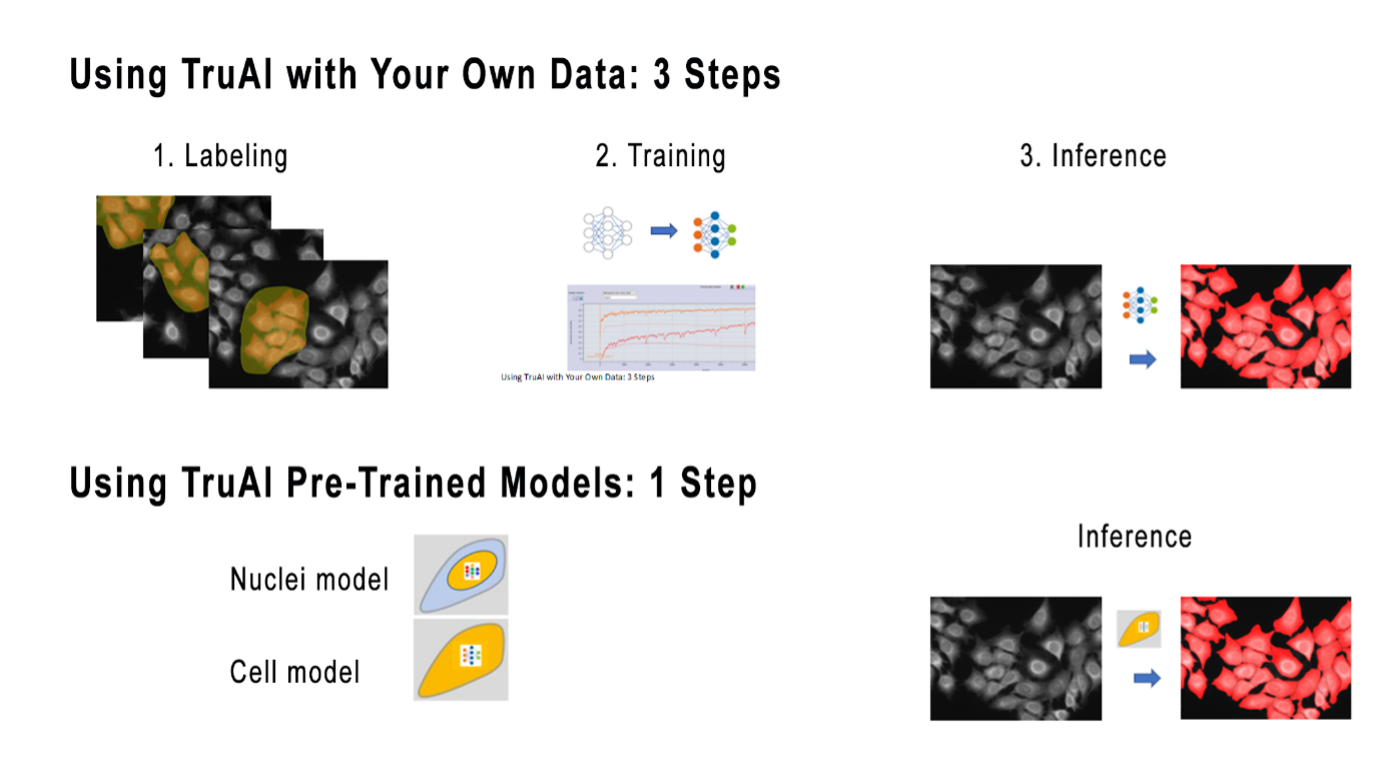
Figure 1. Using TruAI-deep learning technology with your own application data for training and inference helps ensure optimal performance. For a faster approach, pretrained TruAI models enable you to perform the analysis in just one click.
TruAI pretrained models for nuclei and cells are trained to perform instance segmentation in fluorescence images for various scenarios, including a range of:
- Microscopy modalities (point scanning confocal, spinning disk confocal, widefield)
- Stainings (DAPI, phalloidin, MitoTracker, Lamin, etc.)
- Cell lines (HeLa, U2OS, SK-HEP-1, plant cells, tissue, etc.)
- Contrast, signal-to-noise ratios, and background levels
- Pixel resolution and magnifications
It is important to note that the pretrained models are general models and can’t perform the segmentation perfectly in all possible situations. To help you better understand their capabilities, we’ve put together a list of cell segmentation scenarios.
20 Examples of Nucleus and Cell Segmentation Using Pretrained Deep-Learning Models
The image examples below (Figure 2, a–t) show the performance of the nuclei (red) and cell (green) TruAI pretrained models in a variety of segmentation scenarios. This includes different cell lines, marker stainings, magnifications, resolutions, contrast, signal-to-noise ratios, and background inhomogeneities. Note that both models can be applied to the same image (Figure 2, p-t). The models can even be applied to nonspecific nuclei (Figure 2, q–t) or cell stainings (Figure 2p, s).
1. U2OS cells, nuclear staining, widefield 20x, medium confluency with cells of different sizes and morphologies
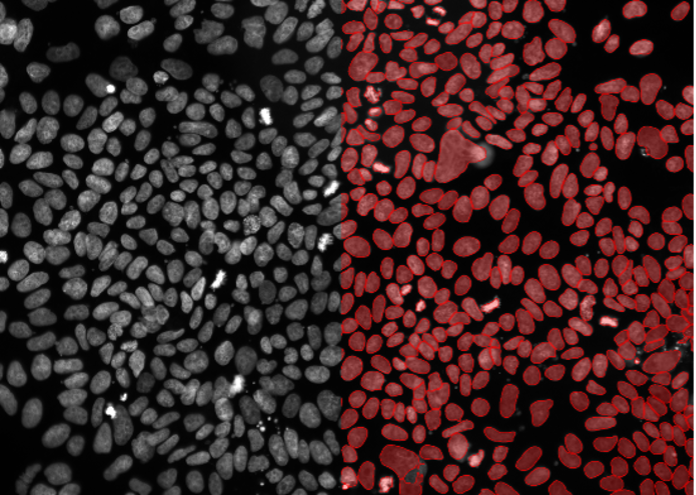
Figure 2a
2. HeLa cells, nuclear staining, widefield 10x, inhomogeneous background
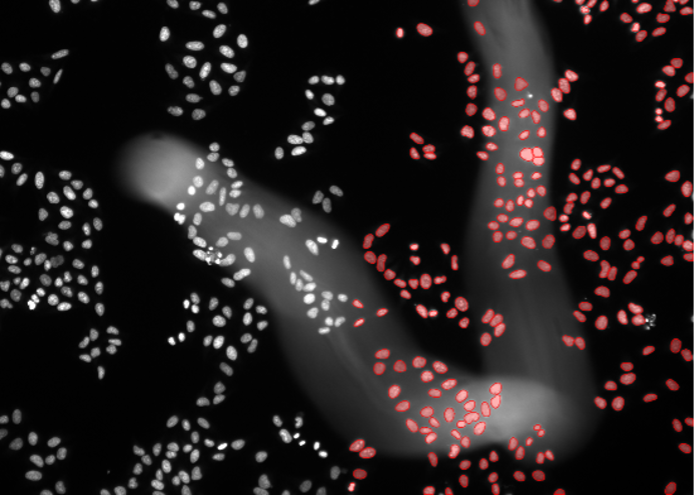
Figure 2b
3. Pluripotenet stem cells, nuclear marker, widefield 20x, very high confluency
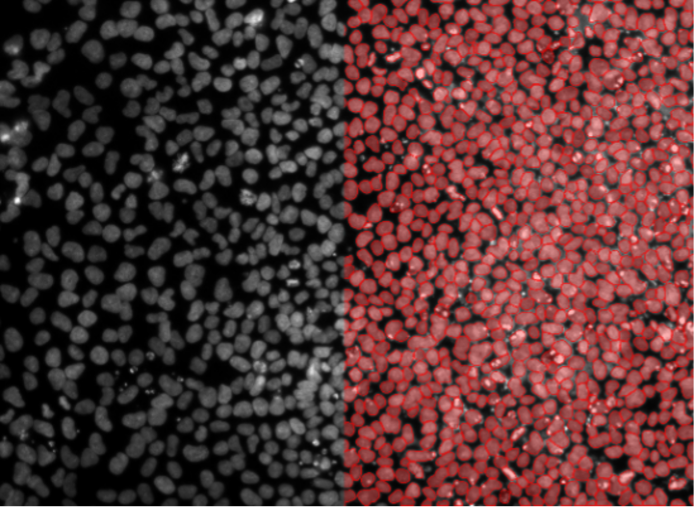
Figure 2c
4. Kidney organoid cells, nuclear staining, confocal spinning disk 30x, high penetration depth
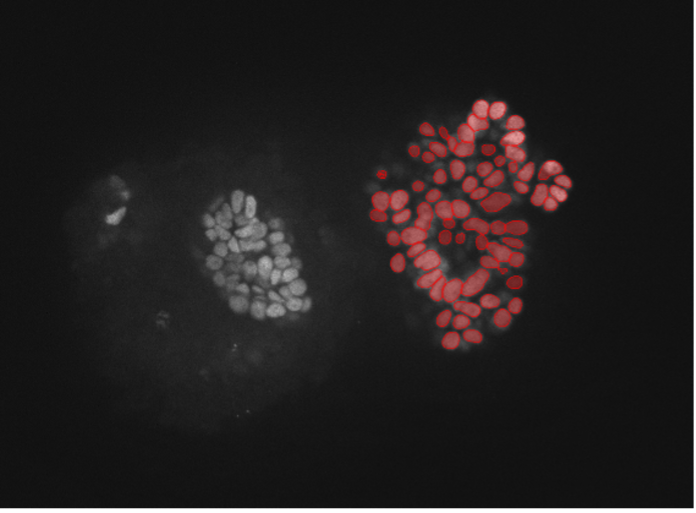
Figure 2d
5. HeLa cells, nuclear staining, confocal spinning disk 40x, high contrast and resolution
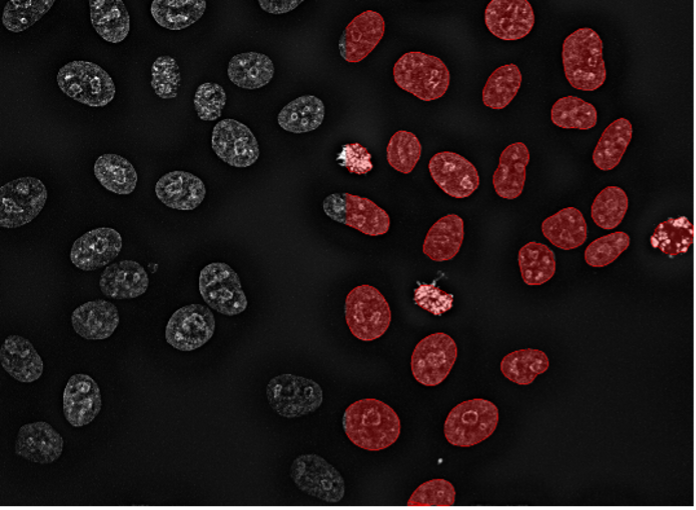
Figure 2e
6. A549 human carcinoma cells, nuclear marker, confocal spinning disk 40x, low signal-to-noise ratio
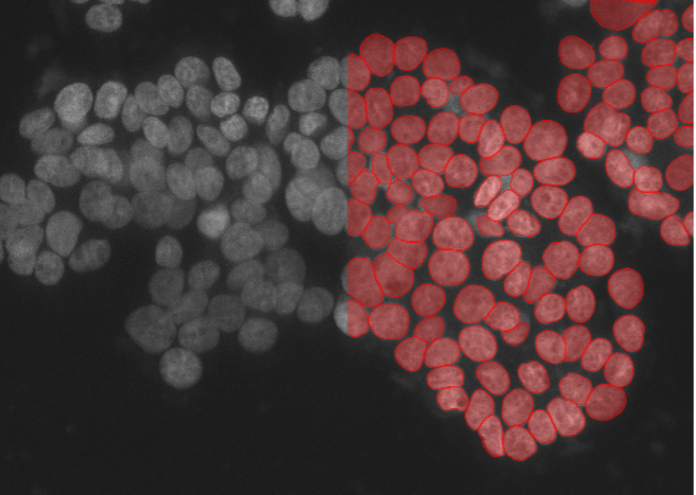
Figure 2f
7. Human tissue tumor cells, nuclear staining, widefield 20x
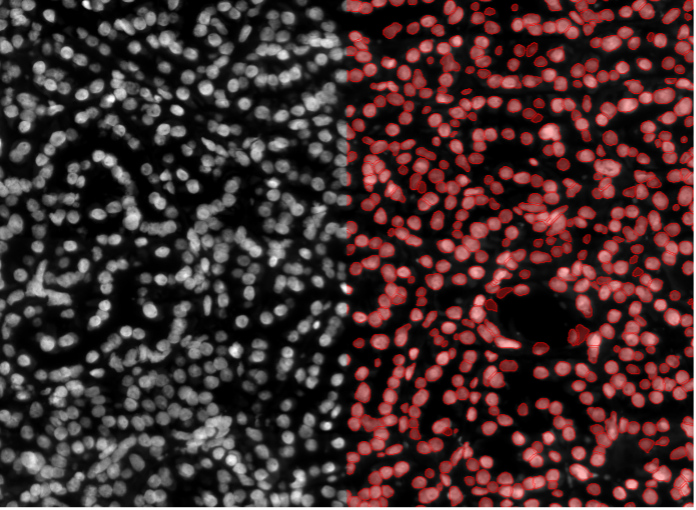
Figure 2g
8. Mouse kidney tissue cells, nuclear staining, widefield 20x, thick specimen
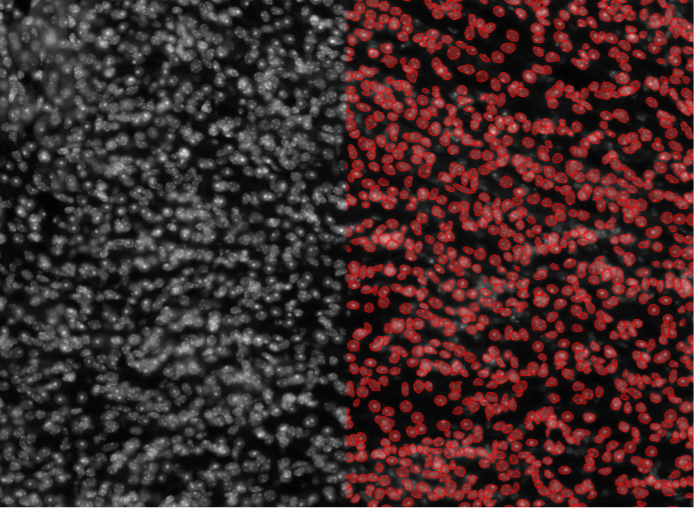
Figure 2h
9. Rat-1 cells, cytosolic staining, widefield 10x, high cell confluency
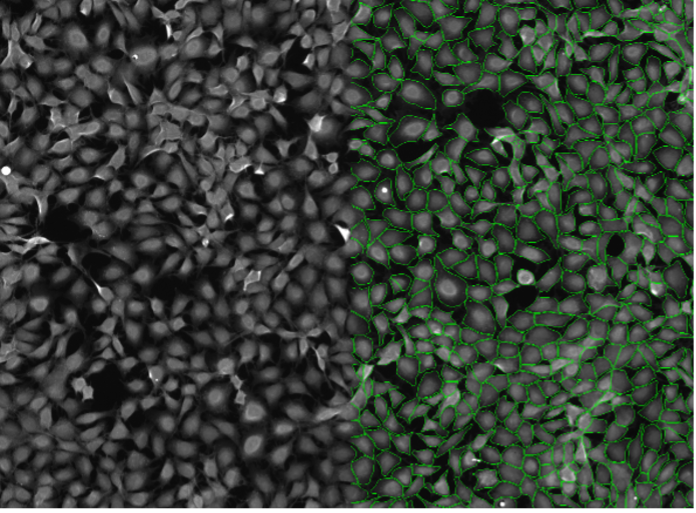
Figure 2i
10. SK-HEP-1 cells, cell contact staining, widefield 20x
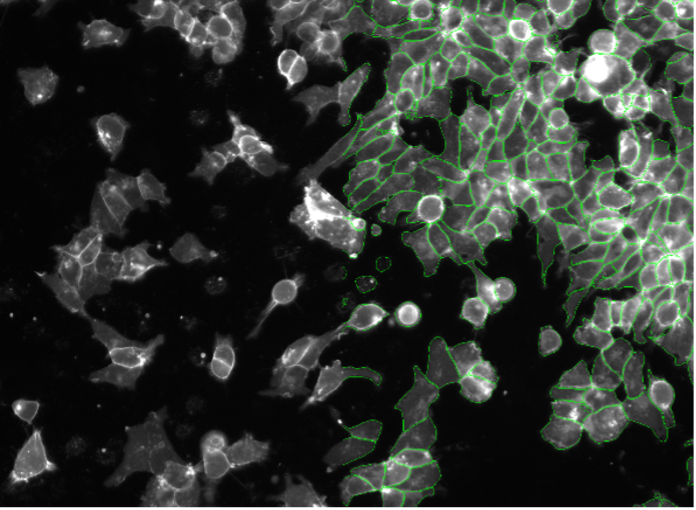
Figure 2j
11. Plant cells, autofluorescence, widefield 20x, low signal-to-noise ratio
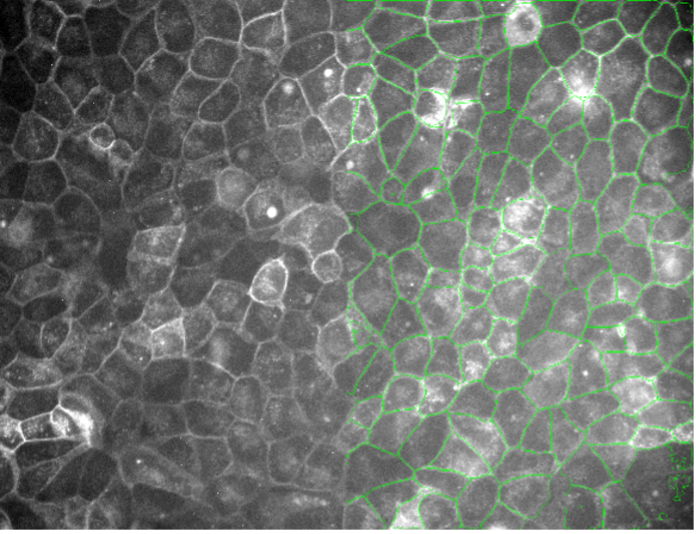
Figure 2k
12. HeLa cells, nuclear membrane staining, widefield 20x
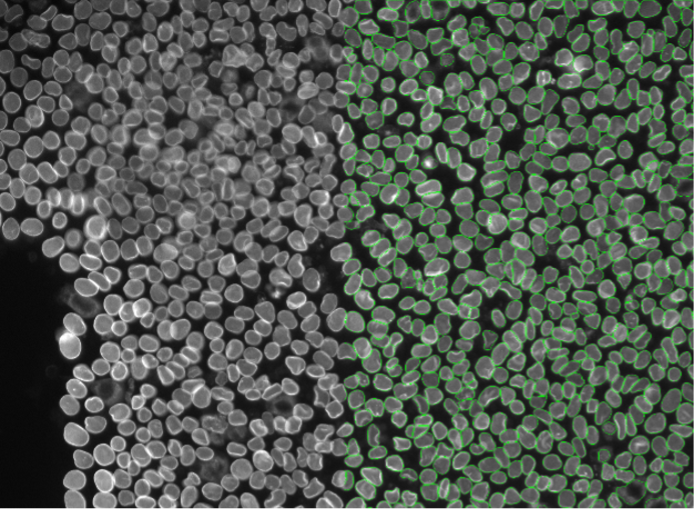
Figure 2l
13. huFIB cells, tubulin staining, widefield 20x
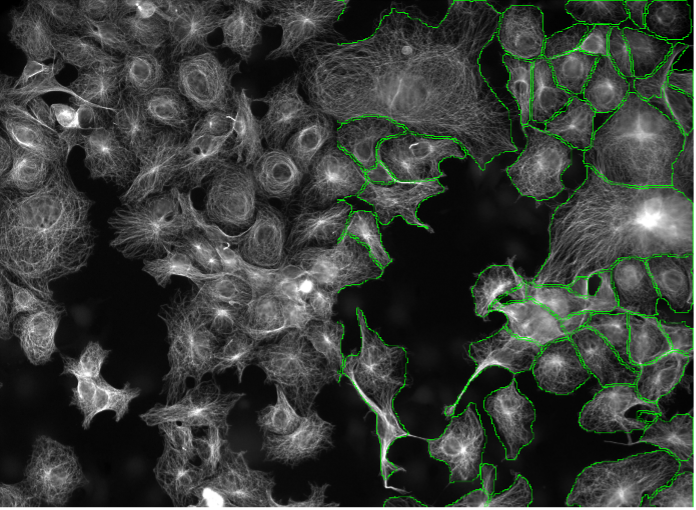
Figure 2m
14. Rat-1 cells, actin staining, widefield 20x
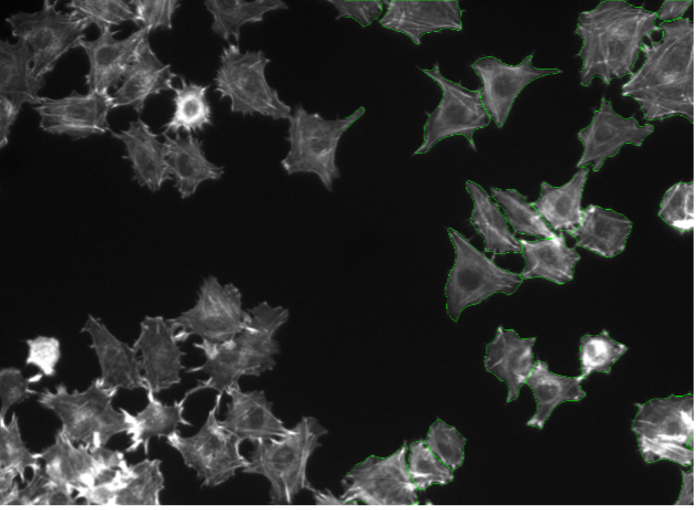
Figure 2n
15. BPAE cells, actin staining, point scanning confocal 40x
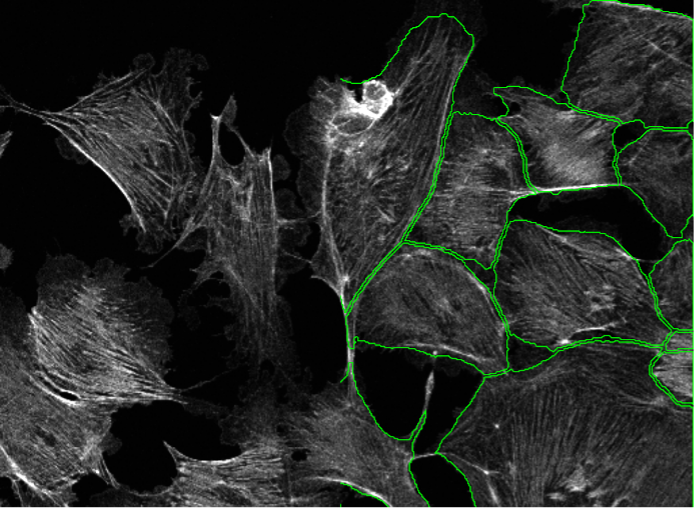
Figure 2o
16. Rat-1 cells, nuclear staining weakly bleeding into cytoplasm, widefield 20x
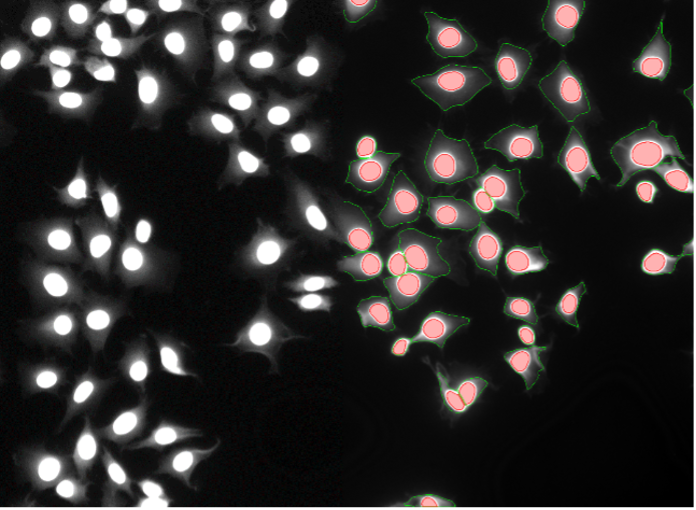
Figure 2p
17. HeLa cells, tubulin staining, widefield 4x, low resolution and low signal-to-noise ratio
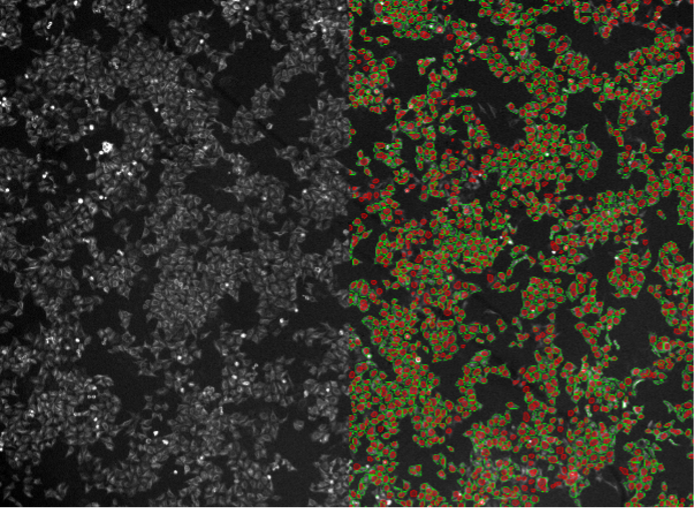
Figure 2q
18. Rat-1 cells, cytosolic staining, widefield 20x
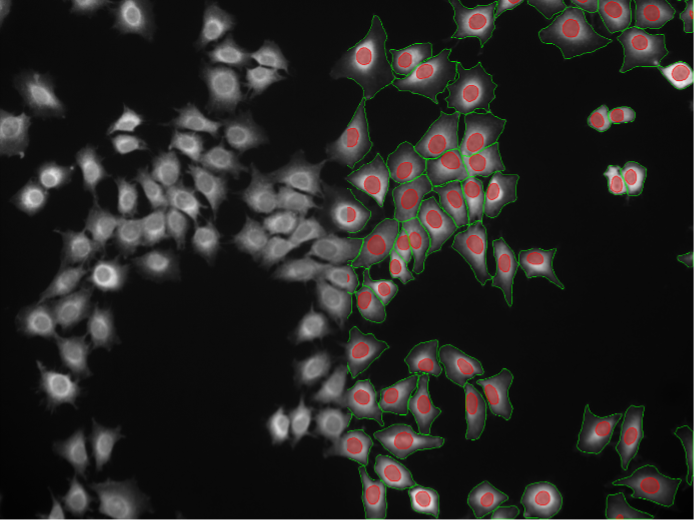
Figure 2r
19. COS-7 cells, mitochondria staining, point scanning confocal 40x
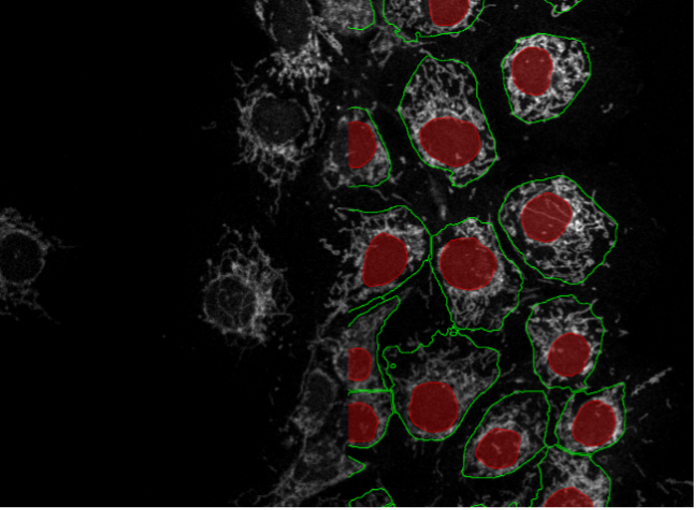
Figure 2s
20. BPAE cells, tubulin staining, point scanning confocal 40x
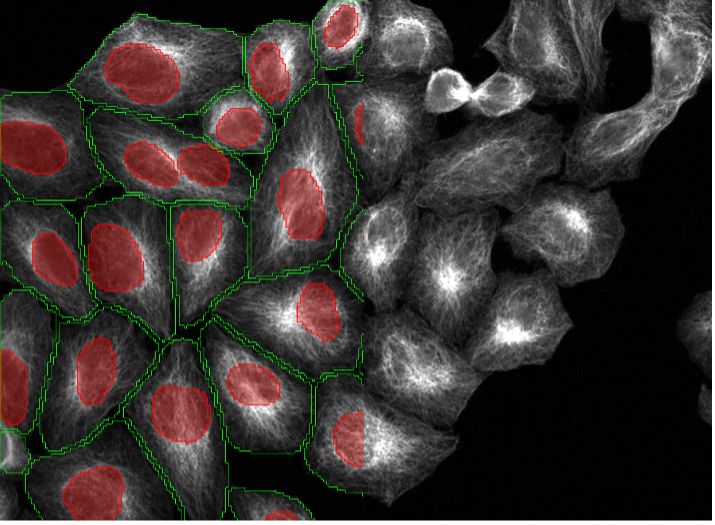
Expanded Deep Learning Capabilities for Cell and Nuclei Segmentation
The introduction of instance segmentation and pretrained models expands the capabilities of the TruAI toolset. Users can now save time by starting with the pretrained models and performing cell and nuclei segmentation in one click. For cases where the results are not optimal, users can convert the TruAI predictions into new labels and then apply corrections. These corrected labels serve as the basis for a new training, saving time and effort.
Our customers are already seeing the benefits of the expanded deep learning capabilites. Robert Strauss, Senior Scientist at the Danish Cancer Society Research Center, commented, “The pretrained nuclei recognition is absolutely stunning and now makes it possible to easily analyze very heterogenous samples without compromising on any cell fractions. Especially in high cell density areas, TruAI-based separation is clearly superior to intensity or edge detection, both speed- and performance-wise.”
Related Content
Instance Segmentation of Cells and Nuclei Made Simple Using Deep Learning
Monitoring Cell Cycle Dynamics During Stem Cell Differentiation Using TruAI Deep-Learning Technology
Webinar: Enhance Your Image Analysis with TruAI™ Deep-Learning Technology