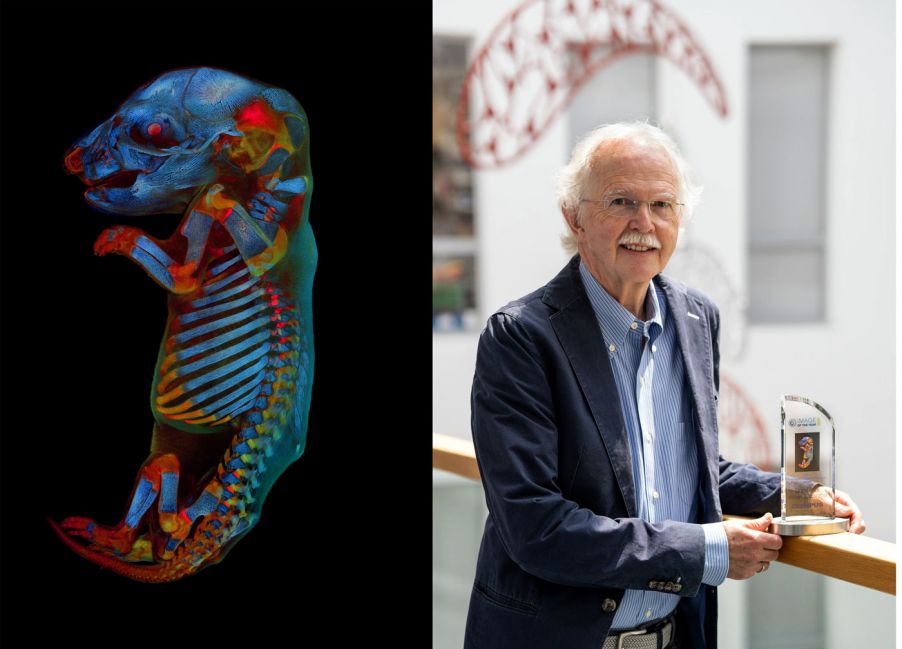
Each year, our Image of the Year (IOTY) Global Life Science Light Microscopy Award recognizes the very best in life science imaging. The winning images are thoughtfully selected by an international jury of experts from both science and the arts.
Over the past few months, we’ve been celebrating the winning images of our IOTY 2020 contest by sharing the stories behind them. So far, we’ve shared stories of the three regional winners for the Americas, Asia Pacific, and EMEA. Today we have one final story to share—the global winner.
Our jury selected Werner Zuschratter from Germany as the global winner for his confocal image of a whole rat embryo. In this colorful and striking image, two channels show different autofluorescence colors of the tissue, while the third channel shows the skeleton stained by alizarin red. We chatted with Werner to learn more about the winning image and get his perspective on the art of science.
Q: Can you tell us about your scientific background?
A: I studied biology with a focus on neurobiology at TU Darmstadt. In 1992, I moved to Magdeburg, Germany, to today's Leibniz Institute for Neurobiology (LIN) and took over the management of a special laboratory for electron microscopy and laser scanning microscopy. The LIN is a research institute for learning and memory with a great need for imaging techniques to explore structural and functional relationships of plasticity at all levels (i.e., from the molecular and cellular level to small animal imaging and human imaging with MRI).
To bridge the various modalities, a couple of years ago we founded an overarching core facility called the Combinatorial NeuroImaging (CNI) Core Facility. I coordinate this facility with a colleague from human imaging. CNI provides infrastructures and training to approximately 150 users per year. The scientists of CNI also conduct their own research projects.
In addition to high-resolution imaging and the observation of dynamic processes, my research interests are focused on the functional analysis of metabolic processes using time-resolved fluorescence lifetime imaging microscopy (FLIM) for nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD) imaging. For this purpose, we developed an ultra-sensitive quantum detector called LINCam.
Q: What does your winning image show?
A: The picture shows a rat fetus that was recorded as individual stacks of images (7 × 13 stacks with 168 focal planes each) using a confocal microscope and then assembled to form an overall picture. The total memory requirement of the original data set is 22.9 GB.
The individual images were recorded sequentially in different spectral ranges (blue, green, and red), with two of the three channels showing tissue autofluorescence and the third channel showing skeletal staining with alizarin red. The sample was originally prepared in a former research project to explore the effects of pharmaceuticals on embryonic development.
I reused the sample to test the quality of clearing methods for whole-body imaging (with confocal or light sheet microscopes), to extract information from autofluorescence in label-free imaging experiments in combination with histochemical staining and to discern different autofluorescent species using advanced spectral and time-resolved microscopy techniques (e.g., FLIM).
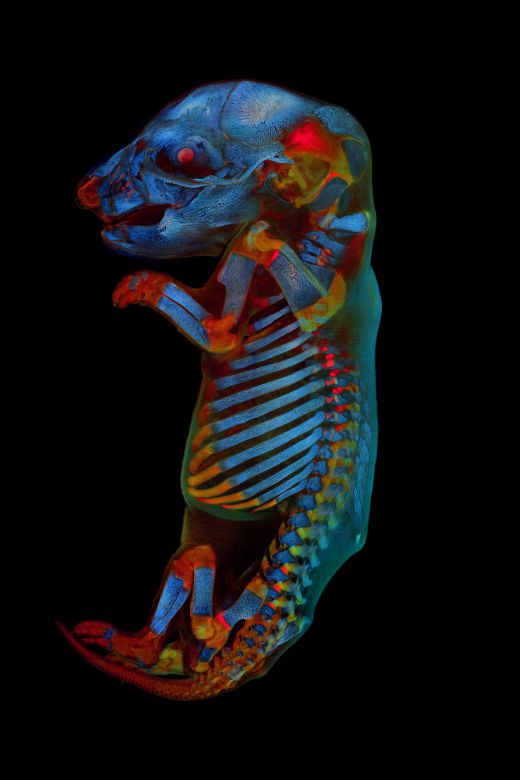
IOTY 2020 global winner Werner Zuschratter captured a 3-channel, large-scale confocal image of a fixed and cleared whole rat embryo.
Below are the technical details for the imaging steps:
Imaging procedure:
- Tile scan of 7 × 13 stacks with 168 focal planes each at 20 µm step size
- Magnification: 5 × 0.15 NA objective
- Grey values: 12 bit
- Image size: 2329 µm × 2329 µm × 3360 µm per stack
- Pixel resolution: 512 × 512 per tile with line average: 3
- Memory requirement: 252 MB per stack; 22.9 GB for all stacks
- Excitation: Channel 1: 405 nm; Channel 2: 483 nm; Channel 3: 568 nm
- Detection: Channel 1: 430–470 nm; Channel 2: 496–541; Channel 3: 641–690 nm
Post-processing:
For post-processing (e.g., Z-projections, merging of stacks, and adjustment of color/contrast), I used ImageJ/Fiji and Adobe Photoshop software.
Q: Why did you choose to submit this image?
A: It is a challenge to capture a widely transparent sample of this size completely in 3D with a microscope and to differentiate tissues by their specific autofluorescence (either by spectral separation or due to their lifetimes).
For this reason, we reused old samples originally prepared for development studies and imaged them using confocal and time-resolved light sheet microscopy. The scans resulted in impressive pictures that emanate something exceptionally aesthetic but also stimulate reflection. Therefore, I concluded that one of the pictures might be eligible for submission to the competition.
Q: What does the art of science mean to you?
A: It is a challenge to capture a widely transparent sample of this size completely in 3D with a microscope and to differentiate tissues by their specific autofluorescence (either by spectral separation or due to their lifetimes).
In this regard, microscopy offers a wealth of opportunities to stimulate creativity and inspiration and to sharpen the eye for new things. For example, three years ago we held an art exhibition with pictures from our users in a gallery to initiate a dialogue with the public about science and art. We also maintain a permanent exhibition for visitors in our institute.
Q: Where and when did you first learn to use a microscope?
A: My interest in microscopic photography was sparked during my studies at TU Darmstadt. At that time, it was common in practical courses to sit at the microscope for days and draw what you saw through the eyepiece, a method that I no longer consider appropriate for data collection. Fortunately, my future boss let me use a research microscope equipped with an analogue camera and automatic exposure metering.
During my diploma and doctoral thesis, I was introduced to the world of electron microscopy. With the advent of confocal microscopes in the 1990s, the improvement of fluorescent dyes, and the introduction of molecular biological labeling techniques, I turned back to light microscopy with an emphasis on dynamic processes using live-cell imaging.
Since functional live-cell imaging requires low doses of light to minimize phototoxicity, our facility has focused on researching ultrasensitive imaging processes based on the principle of single photon counting. This effort has finally resulted in the development of a new camera for FLIM, the LINCam.
Q: What microscopes do you use in your work, and what do you like about using Olympus microscopes?
A: In addition to an electron microscope, our department has numerous fluorescence microscopes. These include several confocal, light sheet, STED, and FLIM microscopes from a wide variety of manufacturers.
What I appreciate about Olympus microscopes is their modular structure, as well as their high transparency and excellent quality optics. For these reasons, we use an Olympus IX81 inverted microscope with TIRF in one of our FLIM setups. Further, one of our light sheet microscopes is based on an Olympus MVX10 zoom microscope. During a recent visit to the Olympus European headquarters in Hamburg, Germany, we tested our LINCam on a SpinSR10 spinning disk confocal microscope and saw the excellent performance and possibilities this combination opens for 3D FLIM applications.
Q: Is there anyone you’d like to thank for their support?
A: First, I’d like to thank the jurors for the high praise that the global IOTY award represents. I also want to thank my team from the CNI Core Facility for their excellent cooperation.
The Search Continues for the Best Light Microscopy Images
The stories behind the IOTY award winning images continue to inspire us to see the beauty found under the microscope. If you have your own microscope images to share, we encourage you to submit your best work (up to 3 images) to our IOTY 2021 contest!
And while you’re there, don’t forget to download our collection of IOTY images to inspire your microscope imaging. Find virtual backgrounds to add a bold backdrop to your online meetings, as well as wallpapers to beautify your mobile and desktop screens.
Related Content
Spreading His Wings: IOTY 2020 Asia-Pacific Winner Shares the Beauty of the Microworld
Lifelong Love of the Microscopic—Meet the IOTY 2020 Americas Regional Winner
Snakeskin on a Microscopic Scale—Presenting the IOTY 2020 EMEA Regional Winner