Super Resolution for All Types of Live Cell Imaging
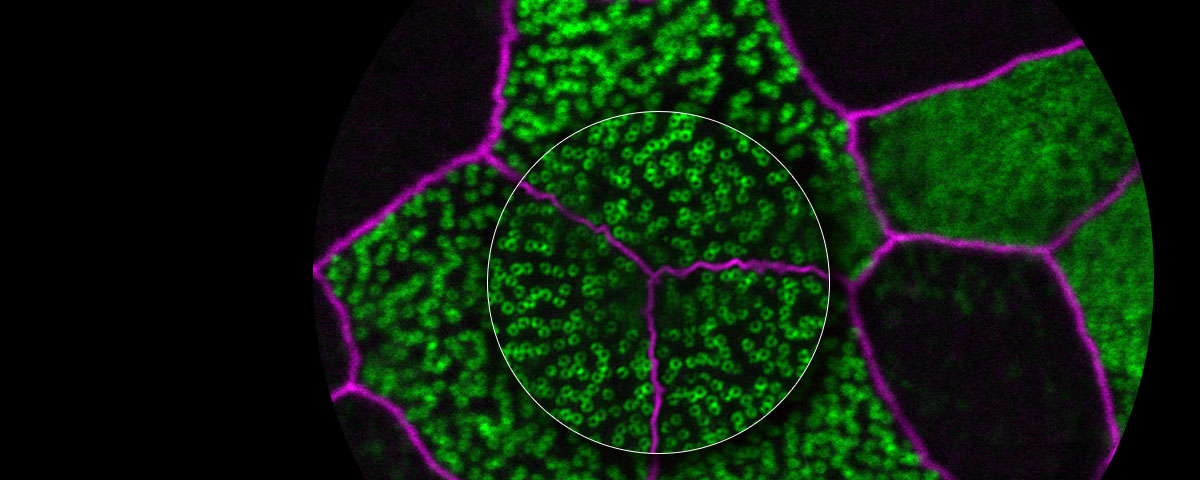
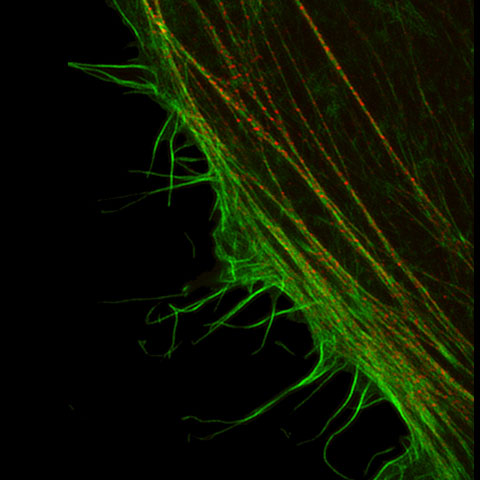
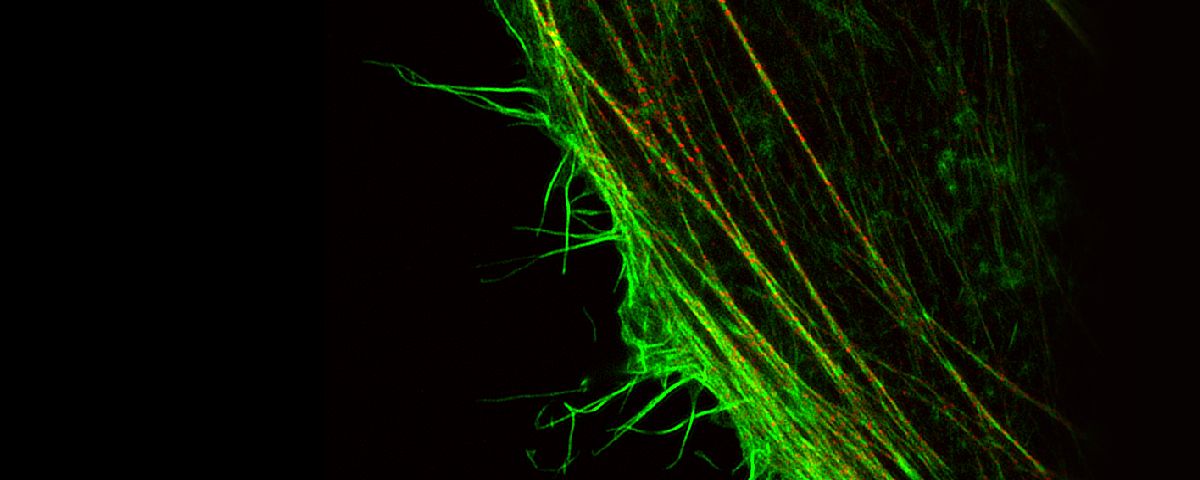
Fluorescence microscopes can identify specific proteins in vivo using fluorescent probes. The resolution of many of these microscopes is restricted by the diffraction limit to about 200 nm, making it impossible to observe fine structures. But with Olympus super resolution technology, you can acquire clear images with a resolution down to 120 nm in the horizontal direction.
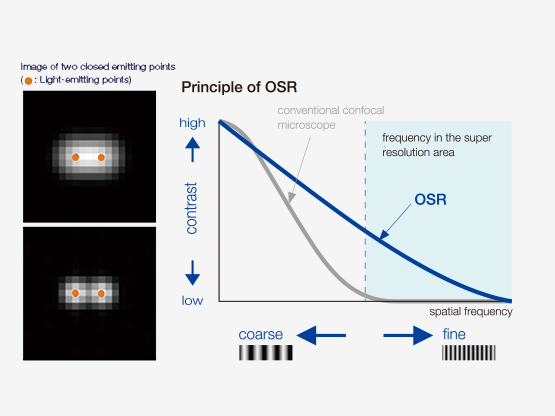
How Does OSR Work?
| Improved detection, specific hardware settings, an optimized confocal aperture diameter, and advanced signal processing, combine to deliver super resolution images. The Olympus Super Resolution (OSR) technology realizes lateral (XY) resolution down to 120 nm. Reference:
|
Olympus Super Resolution (OSR)
IXplore SpinSR10
FLUOVIEW FV3000 with FV-OSR
|
See Even More
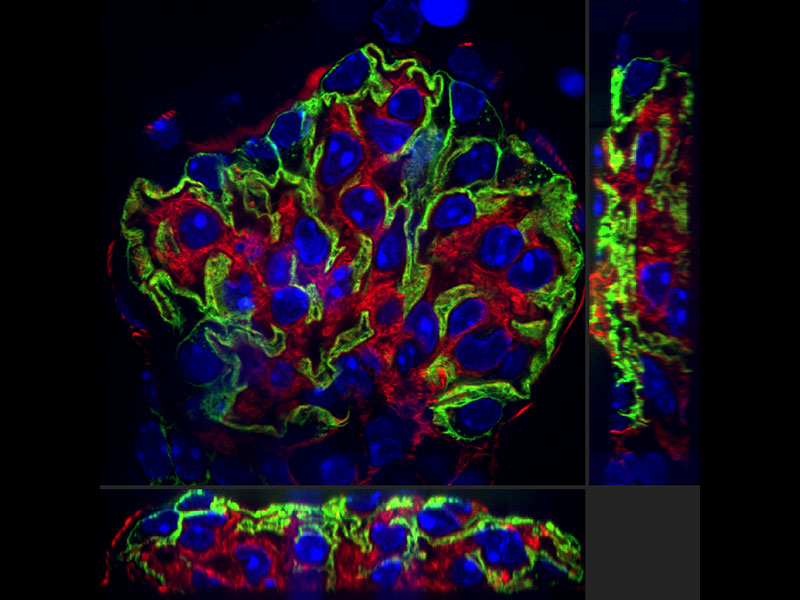
Our deconvolution algorithm makes super resolution images clearer and sharper. The 3D constrained iterative deconvolution removes blur in the Z-axis for a cleaner three-dimensional image.
|
|---|
Testimonials
Yasushi Okada, M.D. and Ph.D., Riken Quantitative Biology Center | Most of intracellular organella and supramolecular complexes are around 100nm size range, and with the regular optical microscopy it was not able to observe the structure of these complexes. In these days, several super resolution microscopy methods are developed, those are not able to try in the field of biology with ease because those methods require dedicated dyes, dedicated observation conditions or special optical systems. This Olympus super resolution microscope is able to upgrade from the spinning confocal microscope easily. Also, the combination with silicone immersion objectives helps to reduce the spherical aberration and able to get the super resolution live imaging in deep plane of cells, I expect this technology will be the tool which able to use in the most of the application in biological field. |
Sachiko Tsukita, Graduate School of Frontier Biosciences and Graduate School of Medicine, Osaka University | We are studying the mechanism behind the coordinated alignment and orientation of basal bodies, which generate cilia in multiciliated cells. The regular basal body-pattern is critical for the metachronal waves of the cilia. To clearly observe GFP-centrin-labeled basal bodies during the differentiation of multiciliated cells in a tracheal primary culture system, a resolution of 200 nm or less is required for each of the X, Y, and Z axes. Using a live-cell imaging system that included the SpinSR10 microscope, it became possible to analyze the movement of the basal bodies. By this method, we discovered a novel mechanism, in which basal bodies align according to the self-organization of the apical cytoskeleton, in multiciliated cells of the trachea. |
Yuji Ikegaya, Ph.D., Laboratory of Chemical Pharmacology, Graduate School of Pharmaceutical Sciences, The University of Tokyo | When I saw the super resolution fluorescence images from the SpinSR10 microscope for the first time, I was surprised by their beauty. I am studying the plasticity of microstructures in the brain, especially the posterior synapse (spine), nerve fibers, and glial cell processes. With the images acquired using the SpinSR10 microscope, I finally realized that I could "see." By using a method to acquire the microstructure in detail other than an electron microscope, the reliability of the acquired data has been improved, and the efficiency of the experiment has been greatly increased due to a simplified data acquisition procedure. Furthermore, the realization of longer time-lapse imaging from the same organization has led to the establishment of new research methods. |
References
S. Hayashi and Y. Okada, “Ultrafast superresolution fluorescence imaging with spinning disk confocal microscope optics,” Mol. Biol. Cell 26(9), 1743–1751 (2015).
S. Hayashi, “Resolution doubling using confocal microscopy via analogy with structured illumination microscopy,” Jpn. J. Appl. Phys. 55(8), 082501 (2016).
A. Nagasawa-Masuda and K. Terai, “Yap/Taz transcriptional activity is essential for vascular regression via Ctgf expression and actin polymerization,” PLoS ONE 12(4), e0174633 (2017).
H. Nakajima, et al., “Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance,” Dev. Cell 40(6), 523-536.e6 (2017).
K. Tateishi, et al., “Three-dimensional Organization of Layered Apical Cytoskeletal Networks Associated with Mouse Airway Tissue Development,” Sci. Rep. 7, 43783 (2017).
E. Herawati, et al., “Multiciliated cell basal bodies align in stereotypical patterns coordinated by the apical cytoskeleton,” J. Cell Biol. 214(5) 571-586 (2016).
M.-T. Ke, et al., “Super-Resolution Mapping of Neuronal Circuitry With an Index-Optimized Clearing Agent,” Cell Rep. 14(11) 2718–2732 (2016).
Sorry, this page is not
available in your country.
Sorry, this page is not
available in your country.
.jpg?rev=2087)