The extensive endoplasmic reticulum network (ER) serves as a factory for the production of almost all of the cell’s lipids. In addition, a major portion of the cell’s protein synthesis takes place on the cytosolic surface of the ER. All proteins destined for secretion and all proteins destined for the ER itself, the Golgi apparatus, the lysosomes, the endosomes, and the plasma membrane are first imported into the ER from the cytosol. Soluble proteins—destined for the ER lumen, for secretion, or for transfer to the lumen of other organelles—pass completely into the lumen. In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of Dronpa fluorescent protein to an endoplasmic reticulum targeting signal.
Video 1 - Run Time: 08 Seconds
Video 2- Run Time: 09 Seconds
Video 3- Run Time: 10 Seconds
To begin their journey along the biosynthetic-secretory pathway, proteins that have entered the endoplasmic reticulum (ER) and are destined for the Golgi apparatus or beyond are first packaged into small COPII-coated transport vesicles. These transport vesicles are in specialized regions of the ER called “exit sites” whose membrane lacks bound ribosomes. In most animal cells, ER exit sites seem to be randomly dispersed across the ER network. The packaging of proteins into vesicles that leave the ER can be a selective process. Some cargo proteins are actively recruited into such vesicles, where they become concentrated. It is thought these cargo proteins display exit (transport) signals on their surface that are recognized by complementary receptor proteins. In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of Dronpa fluorescent protein to an endoplasmic reticulum targeting signal.
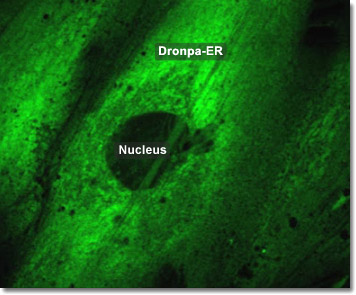
To study the functions and biochemistry of the endoplasmic reticulum (ER), it is necessary to isolate its membrane. This may seem all but impossible given that the ER is intricately interwoven with other components of the cytosol. One way to accomplish this is to disrupt the tissues or cells through homogenization. This causes the ER to break into fragments and reseal into many small closed vesicles called microsomes, which are relatively simple to purify. The ribosomes are always found on the outside surface, so the interior of the microsome is biochemically equivalent to the lumenal space of the ER. In the digital video presented above, embryonic rat thoracic aorta fibroblast cells (A7r5 line) are expressing a fusion of Dronpa fluorescent protein to an endoplasmic reticulum targeting signal.