Cell movement by pseudopodia, a mechanism responsible for motility, depends upon actin filament construction and disassembly. The extension and contraction of the pseudopodium, or “false foot”, moves the cell along the extracellular matrix. On the trailing end of the cell, myosin causes the pseudopodium to contract, which results in a shuffling to the front of the cellular fluid, breaking apart the actin filament network. While the filament network is being rebuilt, the pseudopodium extends out from the cell once again. This is a regular form of travel for many cells, including white blood cells, and is commonly referred to as crawling. In the digital video presented above, several opossum kidney epithelial cells (OK line) expressing a fusion of enhanced yellow fluorescent protein (EYFP) and human beta-actin are recorded using a combination of confocal fluorescence and differential interference contrast (DIC) modes. Note that labeled actin is concentrated in the filaments as well as in lamellipodia ruffles at the edges of the cell.
Video 1 - Run Time: 72 Seconds
Video 2 - Run Time: 59 Seconds
Video 3 - Run Time: 55 Seconds
Video 4 - Run Time: 52 Seconds
Video 5 - Run Time: 62 Seconds
Video 6 - Run Time: 63 Seconds
Video 7 - Run Time: 56 Seconds
Video 8 - Run Time: 55 Seconds
Video 9 - Run Time: 52 Seconds
Video 10 - Run Time: 51 Seconds
Video 11 - Run Time: 49 Seconds
Video 12 - Run Time: 53 Seconds
Video 13 - Run Time: 60 Seconds
In the cells of living organisms, actin filaments are usually organized into large structures by various accessory proteins. The exact structural form that a group of actin microfilaments assumes depends on their primary function and the particular proteins that bind them together. For example, in the core of surface protrusions called microspikes, microfilaments are organized into tight parallel bundles by the bundling protein, fimbrin. Bundles of the filaments are less tightly packed together, however, when they are bound by alpha-actinin or are associated with fibroblast stress fibers. Notably, the microfilament connections created by some cross-linking proteins result in a web-like network, or gel, form rather than filament bundles. In the digital video presented above, several opossum kidney epithelial cells (OK line) expressing a fusion of enhanced yellow fluorescent protein (EYFP) and human beta-actin are recorded using a combination of confocal fluorescence and differential interference contrast (DIC) modes. Note that labeled actin is concentrated in the filaments as well as in lamellipodia ruffles at the edges of the cell.
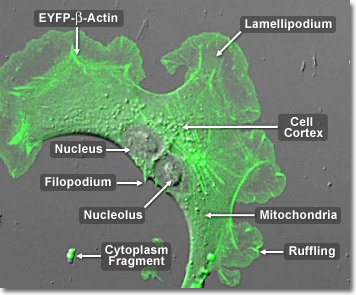
Over the course of evolutionary history of the cell, actin has remained relatively unchanged. This, along with the fact that all eukaryotic cells heavily depend upon the integrity of their actin filaments in order to be able to survive the many stresses they are faced with in their environment, makes actin an excellent target for organisms seeking to injure cells. Accordingly, many plants, which are unable to physically avoid predators that might want to eat them or harm them in some other way, produce toxins that affect cellular actin and microfilaments as a defensive mechanism. The death cap mushroom, for example, produces a substance called phalloidin that binds to and stabilizes actin filaments, which can be fatal to cells. In the digital video presented above, a single opossum kidney epithelial cell (OK line) expressing a fusion of enhanced yellow fluorescent protein (EYFP) and human beta-actin is recorded using a combination of confocal fluorescence and differential interference contrast (DIC) modes. Note that labeled actin is concentrated in the filaments as well as in lamellipodia ruffles at the edges of the cell. After the cell divides, waves of actin can be observed traversing the plasma membrane junction between the two daughter cells.