Actin has the capability of quickly assembling into long polymer rods called microfilaments which have a variety of roles. They interact with myosin to permit movement of the cell, form part of the cell’s cytoskeleton, and they pinch the cell into two during cell division. In muscle contraction, filaments of actin and myosin alternately unlink and chemically link in a sliding action. The energy for this reaction is supplied by adenosine triphosphate, ATP. Actin is present in most cells, especially muscle cells and contributes to the cell’s structure and movement. In the digital video presented above, embryonic rat fibroblast cells (A7r5 line) are expressing a fusion of monomeric Kusabira Orange (mKO) fluorescent protein to human beta-actin.
Video 1 - Run Time: 50 Seconds
Video 2 - Run Time: 57 Seconds
Video 3 - Run Time: 51 Seconds
Video 4 - Run Time: 33 Seconds
Found in all eukaryotic cells (with the exception of nematode sperm), actin is a globular protein and also one of the most high-conserved proteins as it differs by no more than 20% in species as diverse as humans and algae. It is the monomeric subunit of microfilaments—one of the three major components of the cytoskeleton—and of thin filaments, which are part of the contractile apparatus in muscle cells. Actin participates in many important cellular functions, including muscle contraction, cell motility, cell division and cytokinesis and the establishment and maintenance of cell junctions and cell shape. In the digital video presented above, embryonic rat fibroblast cells (A7r5 line) are expressing a fusion of monomeric Kusabira Orange (mKO) fluorescent protein to human beta-actin.
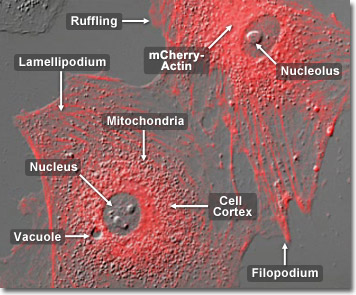
Whether from species as far apart as plants, yeasts and mammals, actins bear a resounding similarity in amino acid sequence. One example of this is actin from Acanthamoeba which differs from mammalian in only 15 out of 375 amino acids, while a comparison of skeletal muscle actin from various species of warm-blooded vertebrates fails to reveal any amino acid change at all. Such resounding evolutionary consistency is extremely rare among proteins. One cited explanation for this is the very large number of protein-protein interactions actin has to fulfill, that it is difficult for such a protein to change its amino acid sequence without adversely affecting one or more of its multiple tasks in the cell. In the digital video presented above, embryonic rat fibroblast cells (A7r5 line) are expressing a fusion of monomeric Kusabira Orange (mKO) fluorescent protein to human beta-actin.



