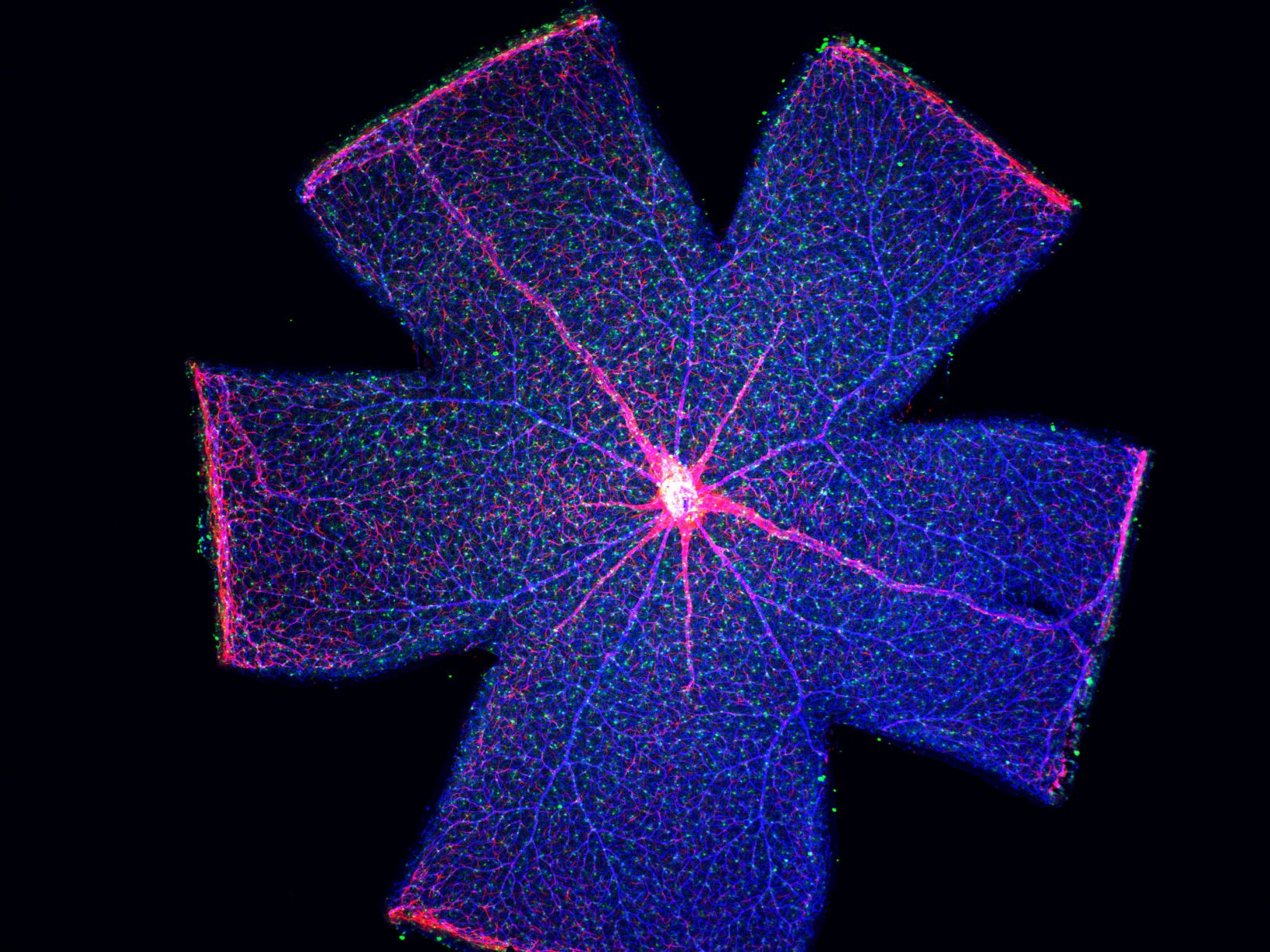
- Introduction
- Imaging Configuration
- TruSpectral
- Macro to Micro Imaging
- Electrophysiology Configuration
- Frame Design
- High Speed Imaging
- Interface for Electrophysiology
- Pre-register button
Introduction
Take a first glimpse at our upright FLUOVIEW systems and preview the possibilities!
Olympus has developed two upright versions of our FLUOVIEW FV3000 microscope for in vitro and in vivo experiments. Now you can take advantage of the speed and flexibility of Olympus' most advanced confocal laser scanning microscope in an upright configuration.
Imaging Configuration
Imaging Configuration
 | Stability for Improved Imaging ReliabilityBy fixing the stage to the frame of the microscope, the BX63LF frame features improved stability for precise imaging. When paired with the highly accurate motorized nosepiece Z drive, users can reliably capture high-precision Z stacks. Easy to OperateThe microscope is equipped with a motorized nosepiece and motorized universal condenser, so the proper optical element is automatically selected in conjunction with the objective. You can attach up to seven objectives to the nosepiece, ranging in magnification from 1.25X to 150X. These features help save time and improve the performance for continuous observations made from low to high magnifications. |
TruSpectral
Multi-Channel Spectral Imaging with TruSpectral Detection
Featuring TruSpectral technology on every detector, the FV3000 system combines high sensitivity with high accuracy for excellent spectral imaging capabilities. *Video: 3D image of chick ciliary ganglion cleared by tissue clearing reagent. Image data courtesy of Dr. Ryo Egawa. Tohoku University Graduate School of Life Science. |
Macro to Micro Imaging
Macro-to-Micro Imaging
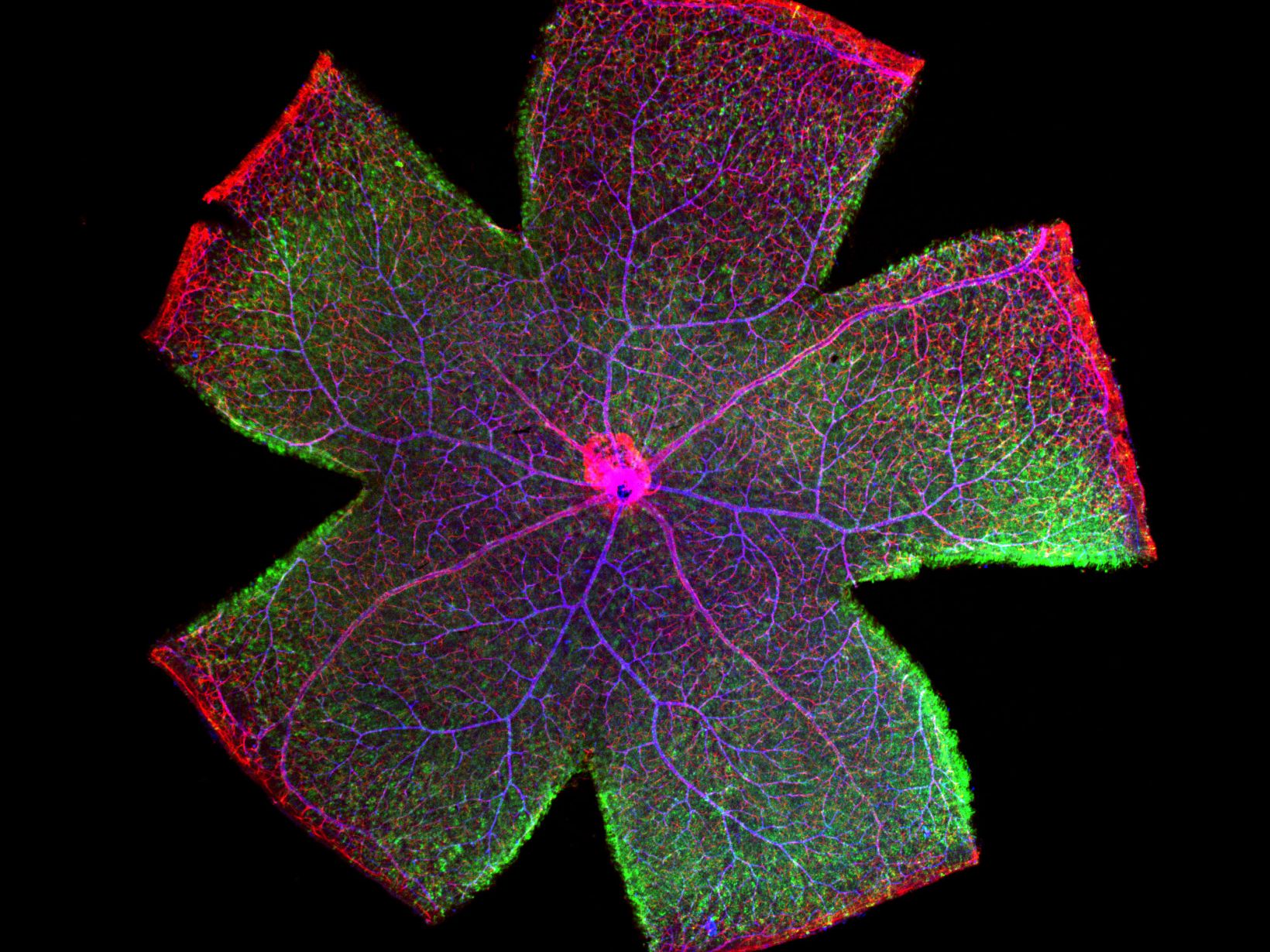
The FV3000 features an optimized macro-to-micro light path to capture images from 1.25X to 150X. Find regions of interest quickly with low magnification overviews, and then switch to higher magnification objectives for high-resolution imaging. Combined with the system’s robust stitching algorithm, you can easily obtain high-resolution macro images that show samples in context. *Image: Mouse retina. One shot overview image acquired by PLAPON2X. Image data courtesy of Dr. Yoshiaki Kubota, The Laboratory of Vascular Biology Center for Integrated Medical Research, School of Medicine, Keio University. |
Electrophysiology Configuration
Electrophysiology Configuration
 | Two Nosepieces for ElectrophysiologyFor electrophysiology applications, you can choose from either a swing or slider nosepiece. The swing nosepiece is compact and swings front to back, enabling users to change water dipping objectives without interfering with the electrodes. The slider nosepiece accommodates one objective with 25 mm diameter threads and a 75 mm parfocal distance, and a second objective with normal (RMS) diameter threads. This nosepiece slides horizontally, making it easy to change objectives while retaining additional working space. Both nosepieces are coded, so the software can tell which objective is currently being used. Senarmont Compensation for DIC ObservationTo help reduce the risk of bumping the stage, specimen, manipulators, or nosepiece when using Senarmont-compensated DIC, the rotatable polarizer has been placed on the base of the microscope frame. The polarizer’s location is also beneficial for IR-DIC to observe cells deep within a brain slice. |
Frame Design
A Suitable Frame for Electrophysiology
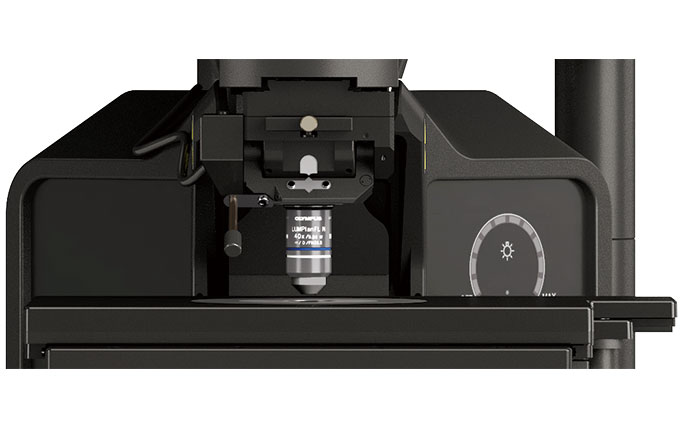 | This configuration offers a larger working space, so there’s more room around the objective for electrophysiology equipment. You can add additional space by lowering the height of the stage to accommodate experiments that involve large samples. |
High Speed Imaging
High-Speed Imaging
Obtain video-rate confocal images with the system’s resonant scanner, without compromising the field of view. Speeds range from 30 frames per second at 512 × 512, all the way up to 438 frames per second by clipping the frame down to 512 × 32. These speeds make the FV3000 particularly suitable for imaging live physiological events such as calcium ion flux. *Video: IMD ratio images of spontaneous Ca2+ oscillation in a beating rat cardiomyocyte expressing yellow cameleon. Image data courtesy of Dr. Yusuke Niino and Dr. Atsushi Miyawaki, Cell Function Dynamics, Brain Science Institute of RIKEN. |
Interface for Electrophysiology
Interface for Electrophysiology
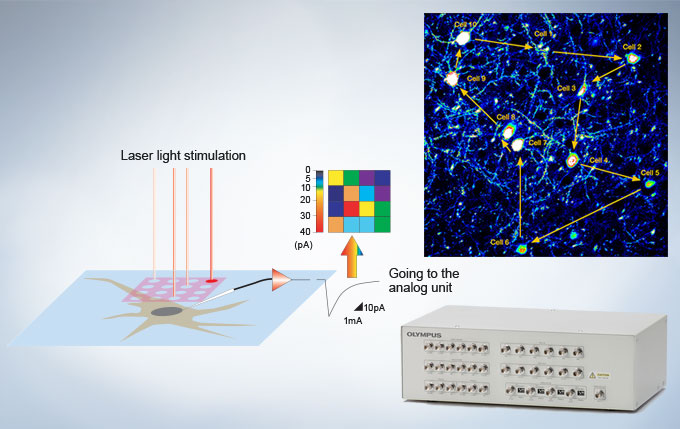 | A trigger signal I/O interface box is available to synchronize confocal imaging events with electrophysiology equipment. The interface box converts voltage signals to images that can be treated in the same manner as fluorescence images, permitting these images to be obtained in synchrony with photostimulation by the confocal scanner. |
Pre-register button
Not Available in Your Country
Sorry, this page is not
available in your country.
.jpg?rev=EE19)