Understanding the Link Between Digital Image Data and Biological Samples
본 백서에서는 생물학 샘플의 신호와 현미경 카메라의 디지털 데이터 사이의 관계를 탐구합니다. 이 관계를 이해하면 최고 품질의 이미지와 데이터를 얻기 위해서 이상적인 이미지 획득 조건을 구성하는데 도움이 될 수 있습니다.
디지털 이미징의 기초
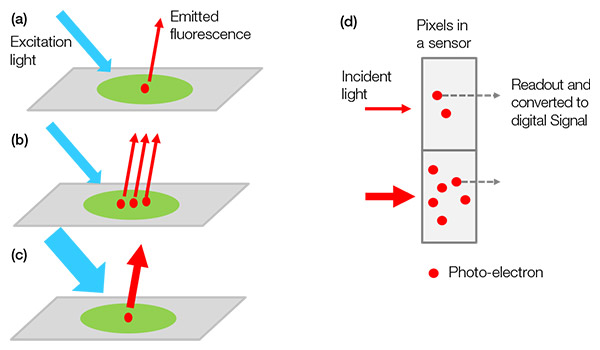
현미경 모노크롬 카메라는 생물학 샘플에서 나오는 빛을 탐지하여 시각화하는 장치입니다. 형광 염료나 단백질에서 방출되는 형광을 현미경이 관찰하면 카메라가 이 빛을 탐지하여 이를 디지털 신호로 검출될 광전자로 변환합니다.
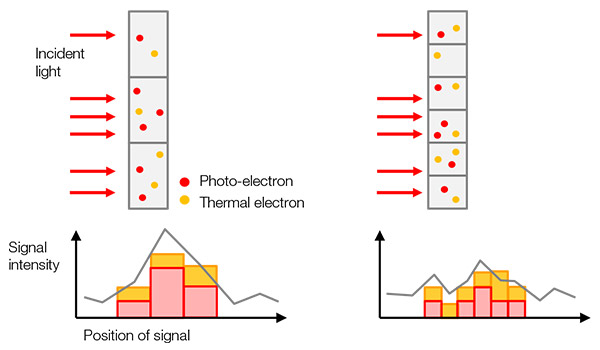
검출된 신호값은 라벨링된 표적(예: 표적 단백질)의 수, 여기광의 강도, 여기광 및 형광 방출/검출의 효율성(빛을 디지털 신호로 전환하는 카메라의 변환 효율 포함)의 곱셈값입니다(그림 1).
하나의 실험에서 각기 다른 샘플에 대해 같은 시스템 및 이미지 획득 설정을 사용하면, 표적의 개수를 제외한 다른 모든 구성요소가 상수가 되어 검출된 신호값이 표적의 개수에 비례하게 됩니다. 이는, 예를 들어, 유전자 편집한 샘플과 그것의 정상형을 정량적으로 비교할 수 있다는 의미입니다.
그림 1- 샘플에서 디지털 신호까지: (a) 라벨링된 표적이 자극을 받아 형광을 방출합니다. |
신호와 배경 노이즈의 구조
여기에서는 이미지의 품질에 영향을 미치는 주된 인자를 설명하고 실험 시 꼼꼼한 이미지 획득의 중요성을 강조합니다.
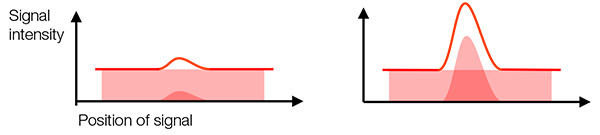
실제 신호와 배경 신호: 검출된 신호에는 실제 신호와 배경 신호(배경 노이즈)가 들어 있습니다. 실제 신호와 배경 신호를 구분하여 표적을 검출하기 위해서는 실제 신호 강도와 배경 신호의 비가 충분히 높아야 합니다(그림 2). 이를 신호-노이즈 비율(SNR)이라고 부릅니다. 높은 SNR을 목표로 삼으면 이미지 품질과 정량분석이 향상됩니다.
SNR을 개선하는 일반적인 방법은 실제 신호를 최대화하고(예: 높은 NA의 대물렌즈 사용) 배경 신호를 최소화(예: 암실, 심층 냉각, 높은 양자 효율 카메라 사용)하는 것입니다.
카메라의 신호 증폭률을 정의하는 게인 설정은 실제 신호와 배경 신호 양쪽에 영향을 미치므로 SNR을 개선하는 효과가 없음에 유의하시기 바랍니다.
그림 2- 왼쪽: 낮은 SNR: 배경 노이즈 때문에 실제 신호를 파악하기가 어렵습니다. |
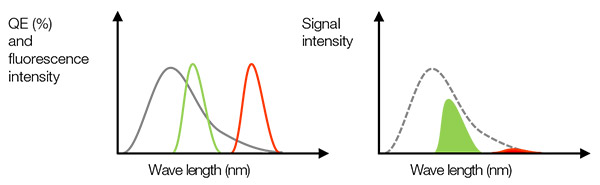
실제 신호: 앞서 언급했듯이, NA가 높은 대물렌즈를 사용하면 SNR을 개선하는데 도움이 될 수 있습니다. 강한 신호를 얻고자 할 때 중요한 또 다른 인자는 높은 양자 효율(QE)입니다. QE는 입사광에서 광전자로의 변환 효율을 나타냅니다. 특정한 파장에서 QE가 0%이면 카메라가 빛을 감지할 수 없다는 것을 기억하십시오. 예를 들어, 생체 조직에서 NIR 창을 확인하거나 복합 중 크로스토크를 예방하기 위해 Cy7과 같은 근적외선(NIR) 염료를 사용하려면 감도가 720nm보다 큰 카메라를 선택해야 합니다.
그림 3- 왼쪽: 회색선은 카메라의 QE입니다. 녹색선과 적색선은 형광 방출 스펙트럼을 나타냅니다. 오른쪽: 검출된 신호값은 면적과 같으며, 이는 왼쪽 그림에서 QE와 형광 스펙트럼의 곱입니다. 이 경우, 형광 강도가 충분할지라도 낮은 QE 때문에 적색 형광에 대해 검출된 신호가 약할 수도 있습니다. |
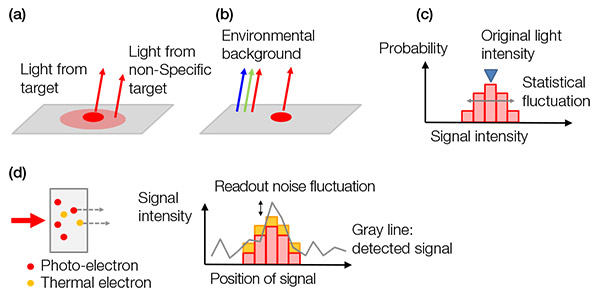
배경 노이즈: 배경 신호는 다음과 같이 분류할 수 있습니다.
a) 생물학적 배경 신호
b) 비생물학적 배경 신호
c) 광전자의 통계적 변동(산탄 잡음)
d) 카메라 내 노이즈
산탄 잡음은 독특하며 동전 던지기에 견줄 수 있습니다. 동전을 두 번 던졌을 때 “앞면”이 나올 확률이 50%이어도 “뒷면”이 나올 수 있는 것처럼 N이 있는 모든 시도에 통계적 변동 ±√(N)이 있습니다. 검출된 광전자의 수 역시 같은 규칙에 따릅니다.
아래의 그림 4는 모든 배경 노이즈의 예를 보여줍니다.
그림 4- 배경 노이즈의 예: (a) 불특정 착색 또는 자발형광에서 나오는 생물학적 배경 노이즈, (b) 슬라이드에 반사된 공간의 주변광, (c) 산탄 잡음, (d) 센서(왼쪽)와 판독 노이즈(오른쪽)에서 발생된 열전자가 있는 카메라 안의 노이즈. 열전자는 센서를 냉각해서 줄일 수 있습니다. |
해상도: 화소 크기가 크거나 비닝(binning) 이미지 획득을 시행하면 더 많은 빛을 캡처할 수 있고 높은 SNR을 제공할 수 있습니다. 그러나 화소 크기가 크면 해상도가 낮아집니다(그림 5). 광학 해상도에 부합하는 최적의 화소 크기를 고려하십시오.
그림 5- 왼쪽: 화소 크기가 크면 감도는 높지만 해상도가 낮습니다. |
현미경 카메라 사용의 모범 사례
이상적인 이미지 획득 설정은 응용 및 샘플에 따라 다르지만, 여기광 강도와 노출 시간이라는 공통의 매개변수가 있습니다. 노출 시간이 길거나 여기광이 강하면 더 밝은 형광이 제공되고, 이는 높은 SNR로 이어집니다. 그러나 광독성이 악화됩니다. 여기에서 중요한 문제 하나가 제기됩니다. 여기광으로 인한 세포 손상을 줄이는 동시에 라이브 셀 이미징 실험을 더 오래 진행하려면 이미지 획득 매개변수를 어떻게 설정해야 할까요?
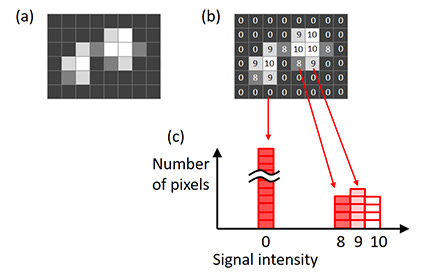
이상적인 노출 시간을 결정하기 위해, 이미지 히스토그램을 사용합니다. 히스토그램의 X축은 신호 강도입니다. 각각의 X값에서 히스토그램의 높이는 신호 강도를 위한 화소수를 보여줍니다(그림 6).
그림 6 – 이미지 히스토그램. (a) 원본 이미지, (b) 원본 이미지에 표시된 각 화소의 신호 강도, |
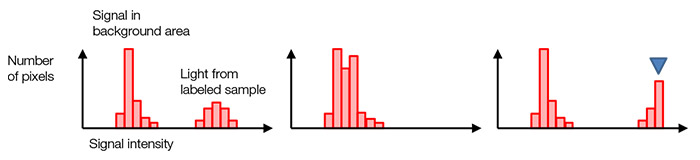
대개, 검정색 배경 화소는 배경광이 전혀 없을 때도 0이 아닌 디지털 신호값을 갖습니다(그림 7, 왼쪽). 이는 그림 4(d)에서 언급했던 판독 노이즈 변동으로 인한 음의 값 신호를 피하는데 도움이 됩니다. 히스토그램의 모양 및 분포는 현재 노출 시간이 적절한지 여부를 알려줍니다. 낮은 신호 범위에서 히스토그램이 너무 밀집해 있다면, 노출 시간이 너무 짧은 것입니다(그림 7, 중간). 최대 신호 수준에 급경사가 있다면, 신호값이 포화 상태입니다(그림 7, 오른쪽). 이 경우 여기 강도를 줄이거나 노출 시간을 단축할 수 있습니다.
그림 7 – 정상 노출 시의 히스토그램(왼쪽), 노출 부족 시의 히스토그램(중간), |
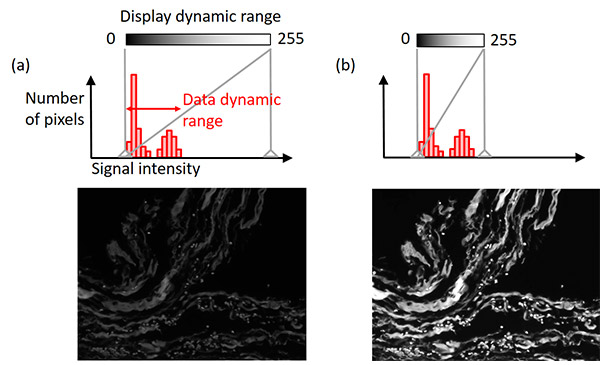
몇몇 이미지 획득 소프트웨어는 자동 디스플레이 조정 기능을 통해 원본 이미지 데이터를 유지하는 동시에 더 우수한 가시성을 제공합니다. 대부분은 모노크롬 카메라의 신호 다이내믹 레인지(예: 16bit = 65,536 레벨)는 디스플레이의 다이내믹 레인지(8bit = 256 레벨)보다 더 넓습니다.
디스플레이 조정 기능은 신호 강도와 디스플레이 밝기 사이의 관계를 정의합니다. 대개는 샘플에서 가장 밝은 신호의 강도는 카메라가 다룰 수 있는 최대 강도보다 훨씬 낮습니다. 이 경우, 디스플레이 다이내믹 레인지를 데이터 다이내믹 레인지(백그라운드 레벨에서 가장 밝은 신호까지의 범위)와 일치시키면 원본 이미지 데이터를 유지하는 동시에 가시성을 향상할 수 있습니다(그림 8). 히스토그램을 통해 이러한 조정이 어떻게 이루어지는지 확인할 수 있습니다.
그림 8 – 디스플레이 조정: (상단) 세로 방향 회색 실선으로 디스플레이 설정 지표를 표시한 히스토그램, (하단) 예시 이미지. 왼쪽 예시 이미지: 원래의 디스플레이 설정. 오른쪽 예시 이미지: 원본 이미지 데이터를 유지하면서 조정한 디스플레이 조건. |
현미경 카메라의 획득 매개변수를 설정하는 6단계
다음은 실험을 위해 현미경 카메라를 적절하게 설정하기 위한 일반적인 6단계를 요약한 것입니다. 최상의 절차는 구체적인 응용 및 샘플에 따라 다르다는 점에 유의하시기 바랍니다.
- 관찰 배율을 결정합니다.
- 샘플의 초점을 조정한 후 관찰 대상을 찾습니다. 프로세스를 단축하고 광독성을 최소화하려면 카메라에서 더 높은 게인이나 비닝(binning) 모드를 사용하시기 바랍니다. 최상의 조건에서 신호를 관찰할 수 있도록 자동 또는 수동 디스플레이 조정을 이용하시고, 이미지를 관찰하지 않을 때는 여기광 셔터를 반드시 닫아두실 것을 권장드립니다.
- 이미지 획득을 위해 다시 게인 및 비닝(binning) 모드를 설정합니다.
- 가장 약한 여기광 강도로 현실적인 노출 시간에 신호를 관찰할 수 있는지 시험합니다. 신호를 파악할 수 없거나 SNR이 너무 낮으면 더 긴 노출 시간을 시도해봅니다.
- 노출 시간이 비현실적으로 길거나 이미지 획득 속도에 허용되는 최대 노출 시간보다 긴 경우, 여기 강도를 단계별로 높이며 시도해봅니다.
- 포화가 없는지 히스토그램을 확인합니다.
결론
현미경 검사 과정에서 이미지와 데이터의 품질에 영향을 미치는 요소는 매우 다양하고 복잡합니다. 그러나 디지털 이미징의 기초 및 팁을 알고 있으면 각각의 실험에 가장 적합한 획득 설정을 판단하는데 도움이 될 수 있습니다. 어떤 응용 및 실험에서든 데이터의 품질을 향상하려면 신호 최대화, 배경 최소화, 샘플 조건 최적화가 필수적입니다.
저자
 |
Takeo Ogama
Scientific Solutions 부서 OLYMPUS CORPORATION OF THE AMERICAS |
Sorry, this page is not
available in your country.