Development of a New Fucci(CA) Application: A Fluorescent Probe for Visualizing Cell Cycles
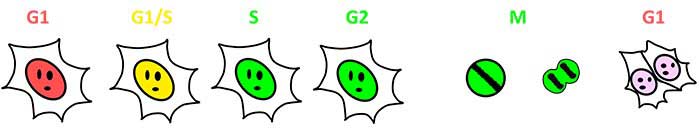
Fucci (fluorescent ubiquitination-based cell cycle indicator) is a genetically encoded set of two fluorescent sensors for live cell-cycle monitoring. Fucci (CA), newly developed in 2017, labels nuclei with either mCherry (red) or mVenus (blue) in a cell-cycle-dependent manner. Fucci (CA) yields abundant expression of mCherry throughout G1 phase with an abrupt decrease in the red fluorescence at the end of G1 phase. Fucci (CA) can be used to reliably detect a short G1 phase and distinguish S and G2 phases, which previously was difficult to achieve.
Fucci (SA) |
|
Fucci (CA) |
|
Figure1: Visualization of Cell-Cycle Progression by Fucci (SA) and Fucci (CA)
Low Phototoxicity, Time-Lapse Image Acquisition of Undifferentiated ES Cells
Undifferentiated embryonic stem (ES) cells rapidly proliferate and are very delicate. Phototoxicity during time-lapse imaging may damage ES cells and reduce their proliferation speed, making it difficult to perform time-lapse imaging of ES cells under physiologically accurate conditions. The FV3000 microscope enables low-phototoxic time-lapse imaging by using extremely low laser power due to the highly efficient lightpath and sensitive detection devices. These properties of the FV3000 enabled a research group to perform a time lapse imaging experiment spanning 57 hours, in which three normal cell cycles of rapidly dividing undifferentiated ES cells were completely covered.
Related Videos |
Figure2: Time-lapse observation of mouse ES cells labeled with Fucci (CA) 2.1
Imaging conditions
Specimen: Mouse ES cells
Objective: Silicone immersion objective (UPLSAPO30XS)
Microscope: FLUOVIEW FV3000 System
Laser: 445 nm (AmCyan), 594 nm (mCherry)
Detection of a Short G1 Phase
Fucci(CA) fully labels the G1 phase with a strong red signal, which enables precise measurement of phase boundaries, even in rapidly proliferating undifferentiated ES cells. Using time-lapse observation at the single cell level, mouse ES cells (mESCs) were found to proliferate with a doubling time of about 11 hours and a G1 phase of only one hour.
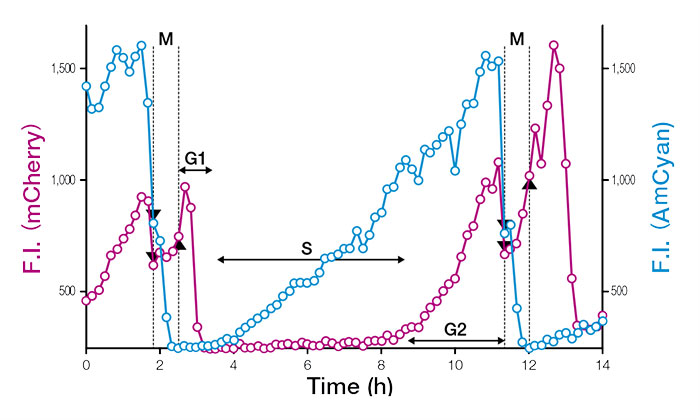
Figure3: Temporal profiles of fluorescence intensities (F.I.) of single cell nuclei expressing Fucci (CA) 2.1
Features of FV3000 enabled this experiment
Fully Spectral System Provides High Sensitivity
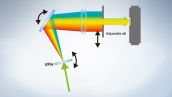
The FV3000 series employs Olympus' TruSpectral detection technology that diffracts light via transmission through a Volume Phase Hologram unit. This technology enables much higher light throughput when compared to conventional spectral detection units with reflection-type gratings.
High Signal-to-Noise Ratio (S/N) Under Low Excitation Light

The high-sensitivity detector unit in the FV3000 has up to four channels of GaAsP photomultiplier tubes (PMTs) that capture weak fluorescent signals with a maximum quantum efficiency of 45%. Additionally, Peltier cooling in these detectors reduces background noise by 20%. Together, these features enable high signal-to-noise ratio imaging, even with low excitation light.
Acquiring Physiologically Accurate Cell Cycle Images: Comment by Dr. Asako Sakaue-Sawano
Undifferentiated mESCs divide and move in a three-dimensional (3D) cell culture space, requiring XYZT imaging to monitor their proliferation. During time-lapse imaging, repeated laser scanning can potentially induce cell-cycle alterations in undifferentiated mESCs due to their high susceptibility to phototoxicity. The FV3000 microscope system permitted Dr. Asako Sakaue-Sawano and her colleagues to perform gentle 4-dimensional (XYZT) imaging experiments to accurately characterize the full cell cycle with very short G1 phase of rapidly proliferating mouse ES cells at the single cell level.
Acknowledgments
This application note was prepared with the help of the following researchers:
Laboratory for Cell Function Dynamics, RIKEN Center for Brain Science
Dr. Masahiro Yo |
Dr. Asako Sakaue-Sawano |
Dr. Atsushi Miyawaki |
Reference
For more details on the studies mentioned in this application note, please refer to the following article:
A. Sakaue-Sawano, et al. “Genetically Encoded Tools for Optical Dissection of the Mammalian Cell Cycle.” Molecular Cell, volume 68, issue 3 (October 2017): pp. 626–640.e5.
Products related to this application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.