全新Fucci(CA)蛋白:用于细胞周期可视化的荧光探针
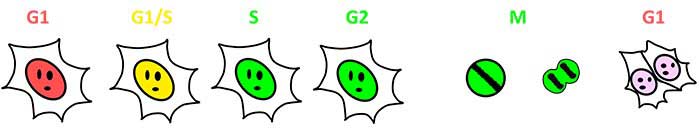
Fucci(基于荧光泛素化的细胞周期指示剂)是一种用于实时细胞周期监测、具有两个荧光探针的基因编码蛋白。2017年新开发的Fucci(CA)蛋白使用mCherry(红色)或mVenus(蓝色)标记不同细胞周期的细胞核。Fucci(CA)蛋白的红色荧光mCherry可以在整个G1期间大量表达,并能在G1期结束时迅速降低,从而可以准确检测短暂的G1期并区分S期和G2期,在过去如此精细的细胞周期区分是很难实现的。
Fucci (SA) |
|
Fucci (CA) |
|
图1:Fucci(SA)和Fucci(CA)实现细胞周期进程可视化
未分化胚胎干细胞的低光毒性时间序列成像
未分化胚胎干细胞的增殖速度很快而且细胞很敏感脆弱。成像过程中光毒性问题很可能造成胚胎干细胞损伤,并降低细胞增殖速度,因此在胚胎干细胞延时成像中很难维持同等的生理环境。针对这类应用,FV3000共聚焦显微镜通过高效的光路和高灵敏的检测设计,以极低的激光功率实现低光毒性的长时程成像。FV3000的这些特性让研究小组成功获得长达57个小时的延时成像,涵盖了未分化胚胎干细胞快速分裂过程中三个完整的细胞周期。
Related Videos |
图2:带Fucci(CA)2.1标记的小鼠胚胎干细胞的时间序列观察
成像条件
样本:小鼠胚胎干细胞
物镜:硅油物镜(UPLSAPO30XS)
显微镜:FLUOVIEW FV3000共聚焦显微镜系统
激光:445 nm(AmCyan),594 nm(mCherry)
对短G1期的检测
Fucci(CA)利用很强的红色荧光对G1期全面标记,即便在迅速增殖的未分化胚胎干细胞中也能精确测量分裂时期的边界。通过单细胞水平的时间序列观察发现小鼠胚胎干细胞(mESCs)的增殖时间约为11小时,而G1期仅为1小时。
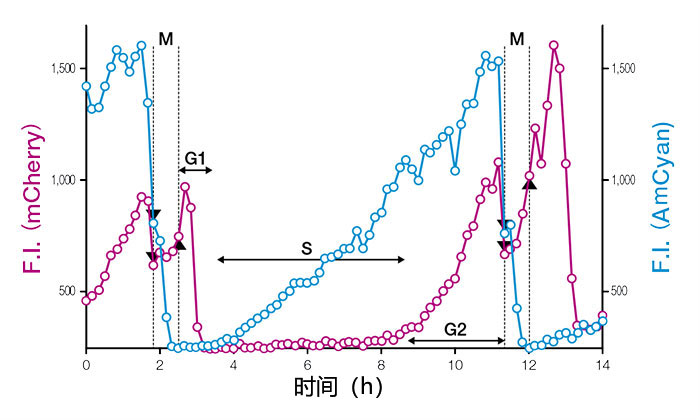
图3:表达Fucci(CA)2.1的单细胞细胞核荧光强度(F.I.)的时间变化曲线
FV3000共聚焦显微镜如何助力我们的实验
全光谱系统实现高灵敏检测
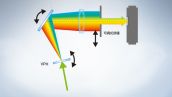
FV3000共聚焦显微镜采用奥林巴斯全真光谱TruSpectral检测技术,通过体相位全息透射光栅提高检测效率。与采用反射型光栅的传统光谱检测单元相比,该技术可实现更高的光通量。
低激发光下的高信噪比(S / N)

FV3000的高灵敏检测单元最多可配四个GaAsP高灵敏检测器,以高达45%的量子效率捕获微弱的荧光信号。此外,配套的Peltier固体制冷技术可将背景噪声降低至20%。这些特点确保FV3000即便在低激发光下也能够实现高信噪比成像。
采集具有生理准确性的细胞周期图像:Dr. Asako Sakaue-Sawano的点评
我们一般使用XYZT成像检测未分化的小鼠胚胎干细胞(mESC)在三维空间中的分裂和迁移。由于未分化的小鼠胚胎干细胞对光毒性高度敏感,延时成像过程中激光反复扫描可能会诱导细胞周期发生改变。FV3000共聚焦显微镜系统帮忙我们实现温和的4维(XYZT)成像,观察到小鼠胚胎干细胞的在极短的G1期下实现快速增殖,并在单细胞水平对整个细胞周期实现准确表征。
致谢
本应用指南的编写得到以下研究人员的协助:
Laboratory for Cell Function Dynamics, RIKEN Center for Brain Science
Dr. Masahiro Yo |
Dr. Asako Sakaue-Sawano |
Dr. Atsushi Miyawaki |
参考文献
有关本应用指南相关研究的更多详细信息,请参考以下文章:
A. Sakaue-Sawano, et al. “Genetically Encoded Tools for Optical Dissection of the Mammalian Cell Cycle.” Molecular Cell, volume 68, issue 3 (October 2017): pp. 626–640.e5.
适于这类应用的产品
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
对不起,此内容在您的国家不适用。