Automating the Drug Screening Process—from Cancer Spheroid Preparation to 3D Imaging Analysis
Summary
A solution combining Yamaha’s CELL HANDLER™ automated cell selection and imaging system with our FLUOVIEW™ FV3000 confocal laser scanning microscope and NoviSight™ software makes it possible to automate nearly the entire cancer spheroid drug screening workflow (Figure 1). This includes operations from the sample preparation stage and all the way to 3D image analysis. An automated workflow makes it possible to run assays using a high number of 3D cell models in a stable and efficient manner.
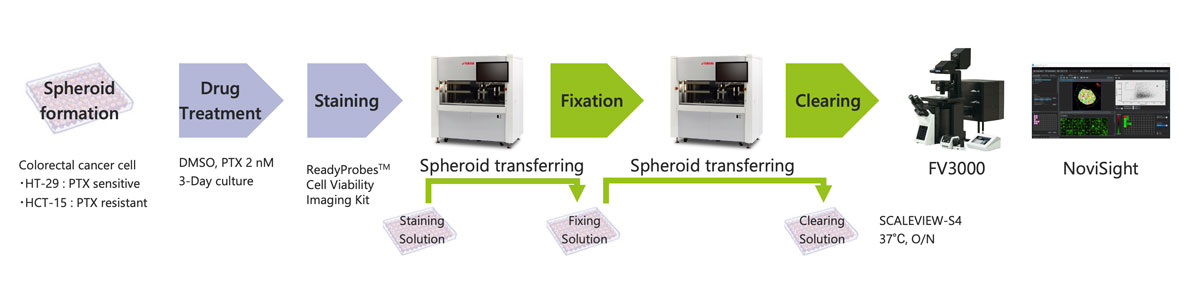
Figure 1. Automated cancer spheroid drug screening workflow
Introduction
Assessing the performance of a drug using three-dimensional cancer spheroids is important because the spheroids reflect the complicated in vivo microenvironment of the cancer much more accurately than two-dimensional models. This enables researchers to evaluate a drug’s effectiveness under conditions that closely resemble a tumor’s microenvironment.
However, the preparation, staining, imaging, and 3D analysis of multiple samples is extremely time-consuming. To simplify this workflow, we have automated spheroid preparation, fixation, and staining with the Yamaha CELL HANDLER™ and image acquisition and 3D analysis with the FV3000 system and NoviSight™ software.
Advantages
- The CELL HANDLER enables sample preparation without cell damage or sample loss. Furthermore, images of spheroids are taken before and after sample transfer, and various parameters are analyzed to help ensure complete traceability.
- The FV3000 confocal laser scanning microscope and NoviSight software can easily perform three-dimensional analysis of multiple samples. It is possible to quantify the differences in the effects of drugs inside and outside the spheroid.
Methods
Cancer spheroid sample preparation
First, spheroids with a diameter of 100 µm were created in an ultra-low attachment U-bottom plate and treated with 2 nM of Paclitaxel (PTX) or DMSO. After a three-day culture, spheroids were stained with the ReadyProbe™ cell viability imaging kit (green/blue) and transferred to a plate with fixing solution and treated overnight at 4 ℃. The cells were then transferred to SCALEVIEW-S4 and treated overnight at 37 °C. All sample transfers were performed with a Yamaha CELL HANDLER.
Imaging spheroids in well plates and 3D imaging analysis
The FV3000 confocal scanning laser microscope was used to acquire multiple fluorescent images of the spheroids on plates. The imaging data was imported into NoviSight 3D cell analysis software, which recognized live and dead cells in the 3D images and then it accurately calculated the viability rates of each well.
Results
Imaging and analysis in the Yamaha CELL HANDLER™
The CELL HANDLER can acquire images and morphology data before and after sample transfer. The images showed that most of the spheroids were successfully transferred without sample loss (Figure 2).
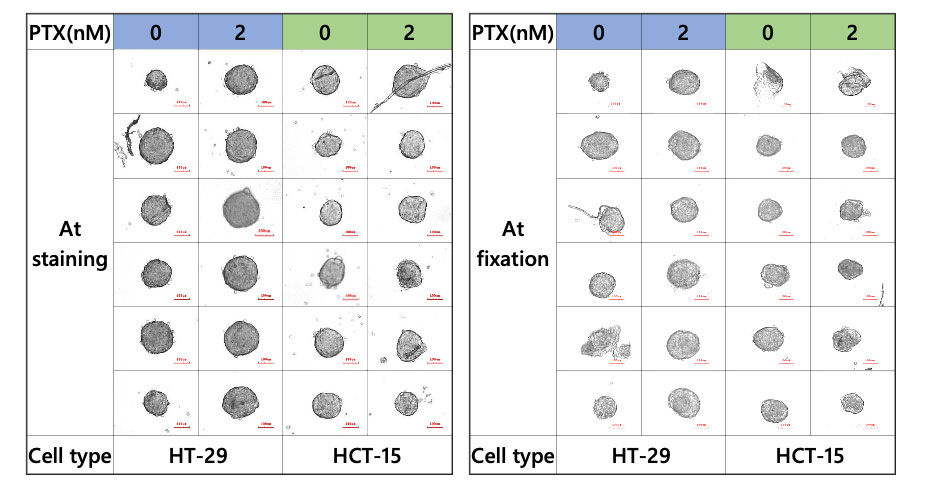
Figure 2. Spheroid during staining and fixation
No difference in area was observed between spheroids treated and not treated with PTX. On the other hand, fluorescent signals showed that PTX significantly reduced the number of live cells in HT-29, while no reduction occurred in HCT-15 spheroids (Figure 3).
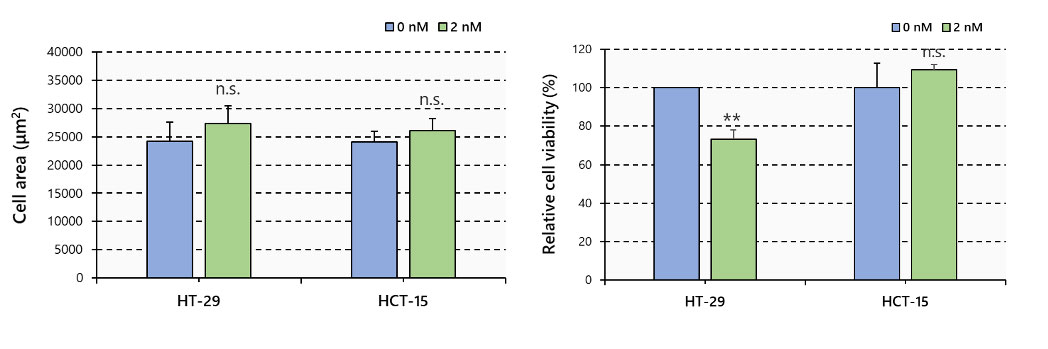
Figure 3. Spheroid area and cell viability, N=6, **: p < 0.01, Error bars: SE
3D image acquisition and analysis with FV3000 CLSM system and NoviSight software
To analyze the effect of PTX on the spheroid microenvironment, the spheroids were observed with the FV3000 microscope (Figure 4).
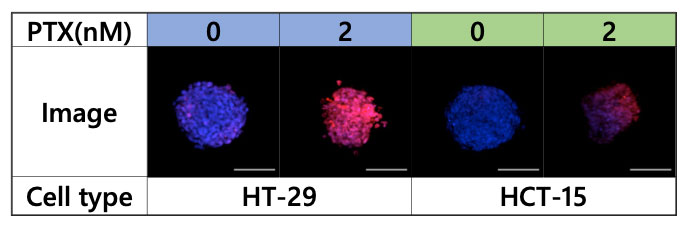
Figure 4. Images acquired using the FV3000 microscope, blue: nucleus (all cells), red: nucleus (dead cell)
Multiple 3D images were then loaded into NoviSight software for 3D analysis, which showed that PTX greatly reduced the number of viable cells in HT-29 spheroids and greatly affected the spheroid from the center to the inside. On the other hand, lesser effect was observed in HCT-15 spheroids (Figure 5).
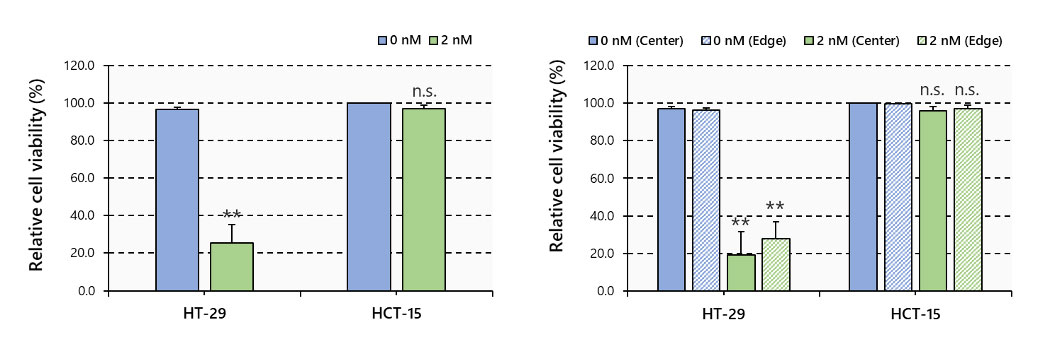
Figure 5. Cell viability by 3D imaging analysis, N=3~6, **: p < 0.01, Error bars: SE
Conclusion
The Yamaha CELL HANDLER simplifies the preparation of large numbers of spheroid samples. The samples can be easily analyzed in three dimensions using the FV3000 microscope and NoviSight software. The accuracy of 3D cell analysis can be improved through the high traceability offered by this automated workflow.
Author
Hiroya Ishihara, Biological Engineering, Research and Development, EVIDENT
Products related to this application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.