Imaging Intracellular Localization of Protein-to-Protein Interactions Using NanoBiT® Technology
A protein-protein interaction (PPI) is the direct physical contact between two proteins. Considering that proteins perform most cellular processes and rarely work alone, studying protein interactions in pairs or larger groups is important across research fields, including drug development and disease research. There are various methods to monitor protein-protein interactions without using cells, but these models do not precisely reflect the intracellular environment. Further, some PPI monitoring methods using cells require lysis and specialized devices for quantitative detection.
To overcome these challenges, Promega developed NanoLuc® Binary Technology (NanoBiT®). NanoBiT® is a structural complementation reporter system that can be used for intracellular detection of PPIs. It has been successfully used in a range of research fields, such as drug screening, analysis of signal transduction, and analysis of the mechanisms of viral infection.
The system is composed of a Large BiT (LgBiT; 17.6 kDa) and a Small BiT (SmBiT; 11 amino acids) subunit, which are fused to proteins of interest. These subunits are then expressed in the cell, so only the targeted PPI causes the subunits to form a functional enzyme that generates a bright luminescence signal (Figure 1).
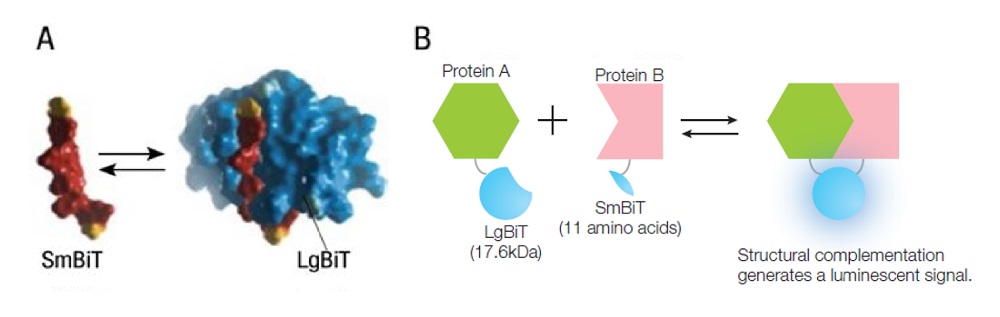
Figure 1. Overview of the NanoBiT® protein-protein interaction system.
Image courtesy of Promega.
Imaging the Intracellular Localization of NanoBiT
To perform cytosolic and nucleic expression, we transfected two kinds of vector pairs into HeLa cells. The first pair was a nontargeted FKBP-SmBiT control vector and FRB-LgBiT control vector. The second pair was a nucleus-targeted NLS1-FKBP-SmBiT control vector and FRB-LgBiT control vector (Figure 2). FKBP and FRB are known to bind under rapamycin treatment.
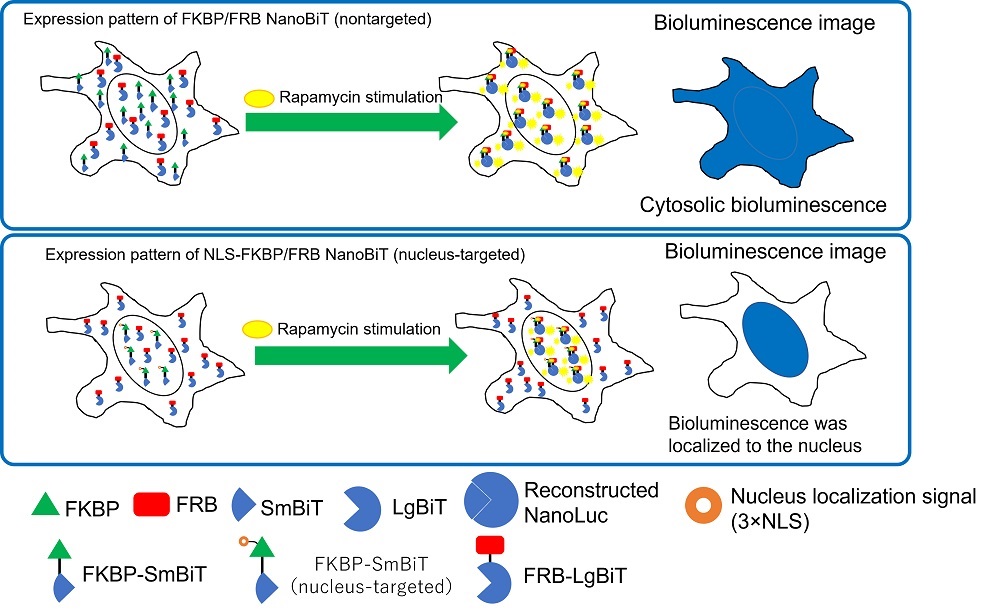
Figure 2. Intracellular localization of FKBP/FRB NanoBiT and NLS-FKBP/FRB NanoBiT.
The bioluminescence from NanoBiT-expressing HeLa cells was then imaged using the IXplore™ Live for Luminescence microscope system2. To confirm the localization of the nucleus, HeLa cells were counterstained with Hoechst33342, and imaged on the same microscope with fluorescence (Figure 3).
We observed the bioluminescence signal in the HeLa cells that received the nontargeted FKBP/FRB NanoBiT to be spread throughout the cytosol. The bioluminescence of the NLS-FKBP/FRB NanoBiT, however, was confirmed to be localized to the nucleus given its overlap with the Hoechst33342 fluorescence stain.
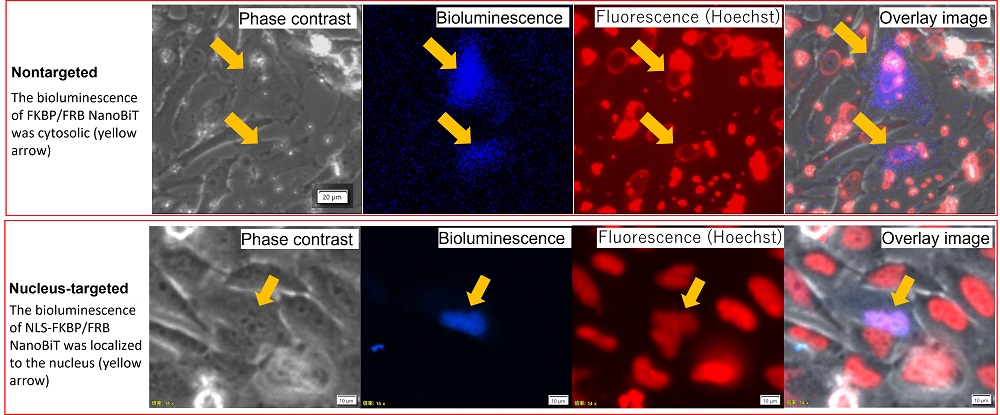
Figure 3. Intracellular localization of FKBP/FRB NanoBiT and NLS-FKBP/FRB NanoBiT.
Experimental conditions:
- Microscope: IXplore Live for Luminescence microscope system2
- Camera: ImagEM EM-CCD camera (Hamamatsu Photonics)
- Objective: LUCPLFLN60XPH, UPLFLN40XPH
- Imaging lens: 0.35X
- EM gain: 1200
- Concentration of furimazine: dilution of 1/200
- Final concentration of rapamycin: 30 nM
- Exposure time: Phase contrast 50 ms / Bioluminescence 3 min / Fluorescence 100 ms
Figure 4 shows the change in the bioluminescence of the NLS-FKBP/FRB NanoBiT before and after the rapamycin stimulation. Bioluminescence intensity in the nucleus increased after the rapamycin stimulation. These results show that FKBP and FRB bind in the nucleus under rapamycin treatment, and the interaction induced the bioluminescence of NanoLuc. Microscopic imaging enabled us to observe the interaction of FKBP and FRB induced by rapamycin stimulation as changes in cytosolic and nucleic bioluminescence intensity in the living cell.
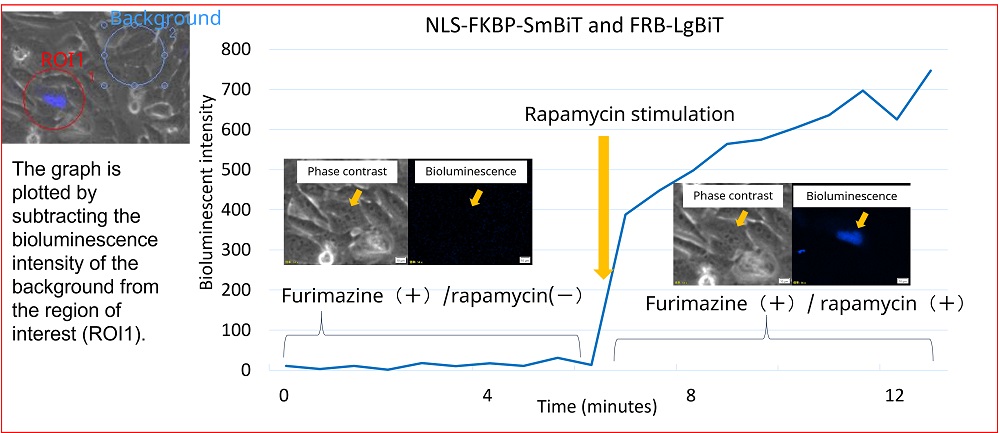
Figure 4. Change in bioluminescence intensity of NLS-FKBP/FRB by rapamycin stimulation.
Experimental conditions:
- Vectors: NLS-FKBP-SmBiT/FRB-LgBit vectors (50 ng each)
- Microscope: IXplore Live for Luminescence microscope system2
- Camera: ImagEM EM-CCD camera (Hamamatsu Photonics)
- EM gain: 1200
- Objective: UPLFLN40XPH
- Imaging lens: 0.35X
- Exposure time: Phase contrast 50 ms / Bioluminescence 30 sec / Fluorescence 100 ms
- Time-lapse interval: 40 seconds
PPI Detection Using a Luminometer and Luminescence Microscopy
NanoBiT and HiBiT3 technologies enable us to detect high-throughput PPIs using a luminometer, while microscopic imaging of those technologies lets us visualize the intracellular localization of those PPIs.
Combining a luminometer with a dedicated luminescence microscope for PPI detection provides several advantages. When observing locally occurred PPIs or changes in their intracellular localization, we can confirm the accuracy of the localization using microscopic imaging before taking a high-throughput measurement using a luminometer. This system eliminates the need for additional fluorescence imaging to confirm the intracellular localization. We can use the same constructed cell line that expresses NanoBiT or HiBiT for checking the intracellular localization and high-throughput luminometer detection.
Author
Taro Hayashi
Researcher, Biological Engineering, Advanced Optics Biological Engineering, Research and Development, Evident
1. Nucleus localization signal (we used 3 times the repeat nucleus localization signal).
2. Bioluminescence imaging system based on the IXplore Live microscope. This system was designed using results jointly developed by Professor Nagai of Osaka University, Japan, Tokai Hit., Co, Ltd. and Evident. This effort was done under the Japan Science and Technology Agency’s program to develop advanced measurement and analysis systems.
3.HiBiT technology for detecting target protein via bioluminescence using an 11-amino acid peptide tag (HiBiT) and approximately 18 kDa NanoLuc luciferase fragment (LgBiT).
Products related to this application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.