Not Available in Your Country
Sorry, this page is not
available in your country.
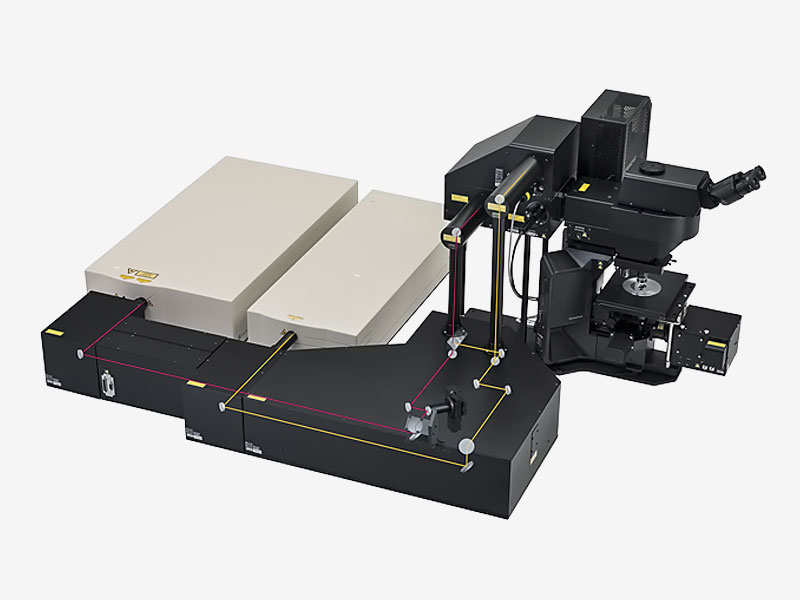
Übersicht
 | Mehr Details in der Tiefe sehenDas FVMPE-RS Multiphotonenmikroskop wurde für Aufnahmen tief in biologischen Präparaten entwickelt und liefert Aufschluss darüber, wie Zellen in lebendem Gewebe funktionieren und interagieren. This product has been discontinued, check out our current product |
|---|
Hochempfindliches, hochauflösendes Deep Imaging mit Multiphotonen-TechnologieDas FVMPE-RS Multiphotonenmikroskop verfügt über fortschrittliche Technologie und ein hochmodernes Optik-Design, um die Empfindlichkeit und Auflösung bei Aufnahmen tief im Gewebe zu verbessern.
|
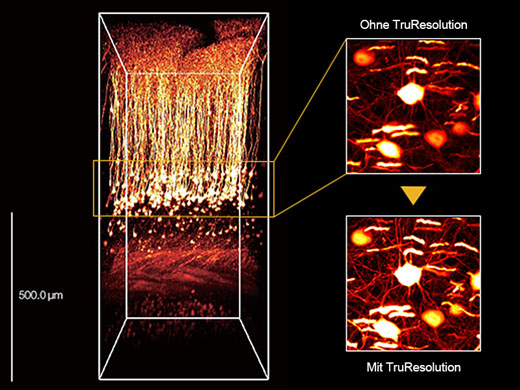
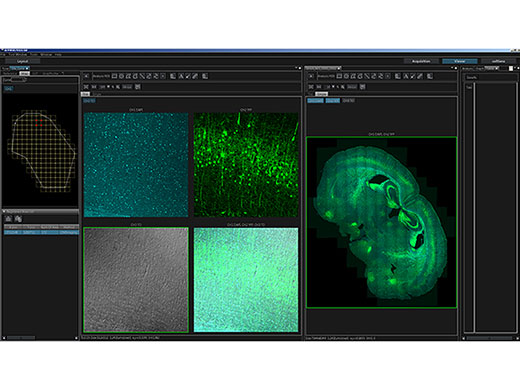
3D-rekonstruiertes Bild eines In-vivo-Maushirns (Thy1-YFP-H-Maus, sensorischer Kortex), aufgenommen mit einem TruResolution-Objektiv mit automatischer Anpassungsfunktion (links) und maximalen Projektionsbildern in einer Tiefe von ca. 600 μm. Bildmaterial ohne (oben rechts) und mit (unten rechts) Verwendung der automatischen Anpassungsfunktion. Die Bilder wurden im RIKEN BSI-Olympus Collaboration Center aufgenommen und von Dr. Hiromu Monai, Dr.Hajime Hirase und Dr. Atsushi Miyawaki zur Verfügung gestellt. |
Related Videos
Blutstrom in einem Zebrafisch-Embryo.
| Hochgeschwindigkeitsbildgebung für schnelle, dynamische zelluläre ProzesseResonanz-Scanning mit hoher Geschwindigkeit und lineare Abtastung mit hoher Auflösung sind Standard.
|
|---|
Breitere spektrale Abdeckung durch Multiwellenlängen-AnregungDie FVMPE-RS Bildgebungsplattform unterstützt einen gepulsten Infrarotlaser mit zwei Wellenlängen oder zwei unabhängige abgleichbare Infrarotlaser für die Multichannel-Bildgebung mit Multiphotonen-Anregung.
|
Bildgebung von Schweinefettgewebe mit Frequenzverdreifachung. Ungefärbtes Schweinefettgewebe wurde mit einem Femtosekundenlaser bei 1250 nm bestrahlt, die Frequenzverdopplung an Kollagenfasern wurde bei 625 nm und die Frequenzverdreifachung an Lipidgrenzflächen bei 416 nm detektiert. |
| Optionale Funktionen für erweiterte AnwendungenDas FVMPE-RS Multiphotonenmikroskop ist eine modulare Plattform, mit der das vorhandene System bei steigendem Einsatz in der Forschung problemlos erweitert werden kann. Verfügbare Optionen:
|
|---|
Für die Multiphotonen-Betrachtung optimierte intuitive SoftwareDas anwenderspezifische Software-Layout bietet mehr Flexibilität und steigert so die Effizienz:
Online-Analyse und Bildverarbeitung, einschließlich spektraler Entmischung und 3D-Rendering, sind Standard. |
|
3 Mikroskopstative zur Auswahl |
Aufrechtes Mikroskopsystem — für In-vivo- und In-vitro-Multiphotonen-MikroskopieDer große Tischabstand und der lange Fokushub des aufrechten Standardstativs bieten Platz für eine Vielzahl von Proben, von Gewebeschnitten bis hin zu lebenden Mäusen und anderen kleinen Tieren. |
Mikroskopsystem mit Schwenkvorrichtung — Für In-vivo-Beobachtungen mit erhöhtem PlatzbedarfDer Abstand von 355 mm zwischen dem Objektiv und der Grundplatte erleichtert In-vivo-Beobachtungen mit großem Platzbedarf, z. B. bei Bildgebung des Verhaltens von Mäusen im Wachzustand. Weitere Informationen über das Mikroskopsystem mit Schwenkvorrichtung |
Invers-Mikroskopsystem — Zur In-vitro-Betrachtung von 3D-Zellen (Sphäroid) und GewebekulturenDas inverse Stativ ist eine stabile Plattform für Zeitrafferaufnahmen dicker lebender Proben, insbesondere von Gewebekulturen und 3D-Sphäroid- und Organoidzellkulturen. Diese Konfiguration ist auch nützlich für die intravitale Bildgebung von Organen und Geweben bei einem kleinen Tier durch ein Körperfenster hindurch. |
Angewandte Technologien
 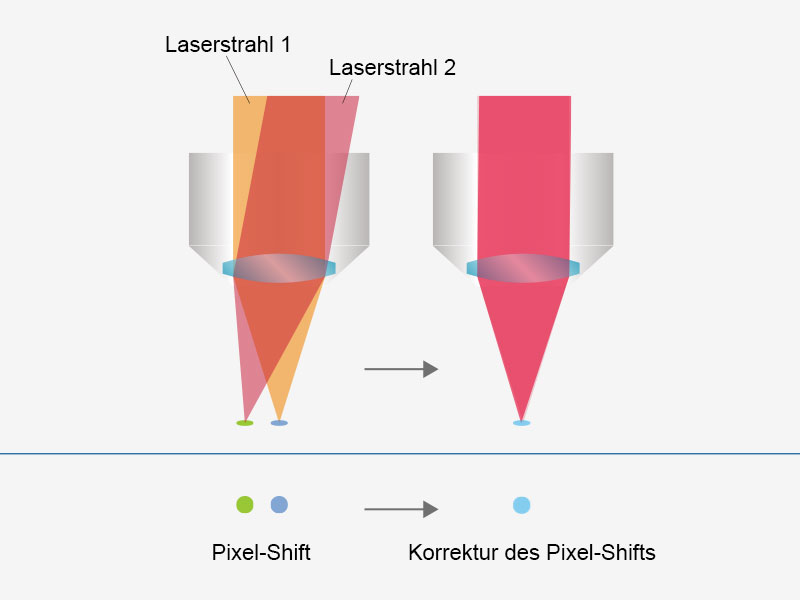 | Anwenderfreundliche, präzise Bildgebung mit automatisierter LaserausrichtungEine durch Wellenlängenabgleich, Temperaturschwankungen und andere Ursachen für Hohlraumverschiebungen verursachte Laserdrift kann in typischen Systemen eine Fehlausrichtung des Anregungsstrahls verursachen. Das FVMPE-RS Multiphotonenmikroskop erhält selbst bei einer Laserdrift eine präzise 4-Achsen-Laserausrichtung des Anregungsstrahls in die Scannereinheit und vereinfacht so die Systemwartung. Strahlposition und Strahlwinkel werden automatisch angepasst, um eine höhere Laserleistung und einheitliche Pixelregistrierung zu erzielen. Wenn das vorhandene System über zwei Anregungslaserlinien verfügt, wird durch diese Funktion die Ausrichtung zwischen den Strahlen beibehalten, und Fehler bei der Registrierung zwischen den Kanälen werden vermieden. |
|---|
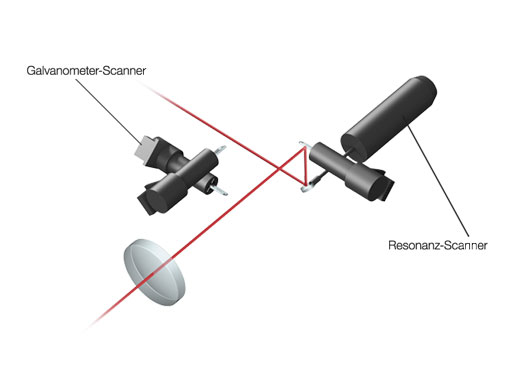
Hochleistungsfähiges Scannen mit bis zu 438 Bildern pro SekundeDer schnelle Resonanz-Scanner und der lineare Galvanometer-Scanner ermöglichen schnelle und hochauflösende Aufnahmen mit ein und demselben System. Vorteile durch die Aufzeichnungsraten von bis zu 438 fps bei 512 × 32 Pixeln oder 30 fps bei 512 × 512 Pixeln über das gesamte Sehfeld (FN 18):
|
|
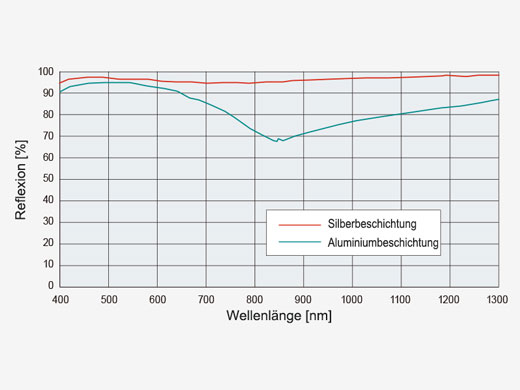
| Effiziente LasertransmissionDurch silberbeschichtete Scannerspiegel wird eine höhere Laserleistung auf das Präparat übertragen, sodass hellere Bilder möglich werden. Die erhöhte Reflexion im nahen Infrarotbereich im Vergleich zu herkömmlichen aluminiumbeschichteten Spiegeln ist besonders vorteilhaft für In-vivo-Untersuchungen in tiefen Schichten. |
|---|
Maximierung der Leistung der Fluoreszenzbildgebung durch DetektoroptionenMit hochempfindlichen Galliumarsenidphosphid (GaAsP) Photomultiplier-Röhrendetektoren (PMT) können Bilder mit hohem Signal-Rausch-Verhältnis (SNR) auch bei schwacher Fluoreszenz aufgenommen werden. Sie bieten eine höhere Quantenausbeute als Standard-Multialkali- (MA-) PMTs und verfügen zur weiteren Verbesserung des SNR über eine lüfterlose Kühlung. Bei hell emittierenden Proben lässt sich das hohe SNR des GaAsP-Detektors mit dem größerem Dynamikbereich des MA-Detektors kombinieren. Um eine höhere Lichtausbeute zu erreichen, verfügt der Non-Descanned Detektionsstrahlengang des Systems über eine großflächige Optik zur besseren Erfassung gestreuter Fluoreszenzphotonen, sodass die breite Erfassungskapazität unserer Multiphotonenobjektive voll ausgeschöpft werden kann. |
|
MPE-Objektive für Deep Imaging
|
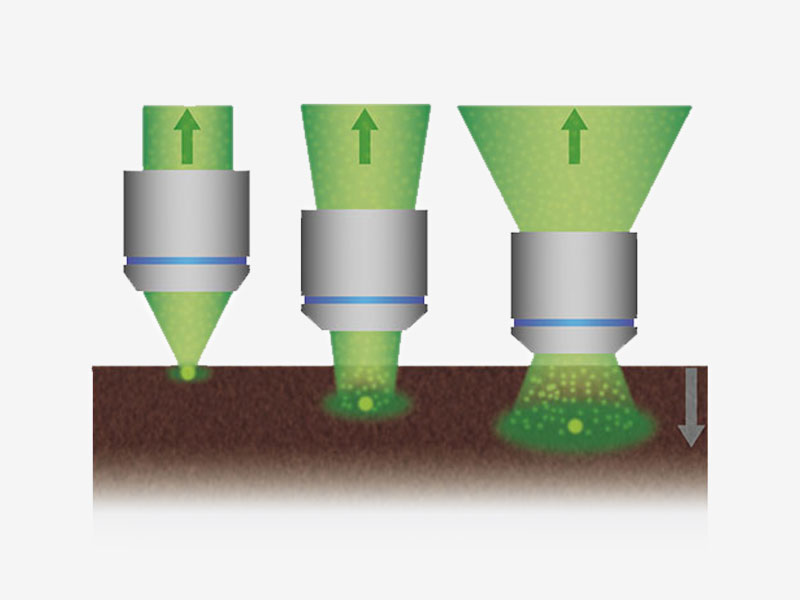
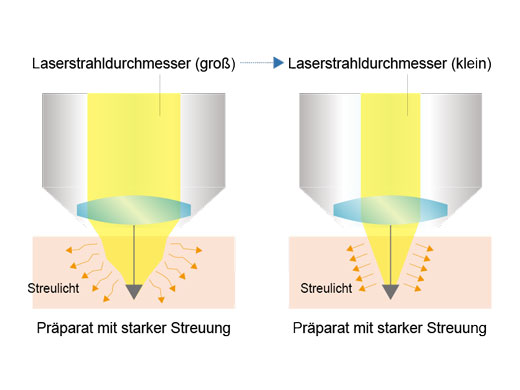
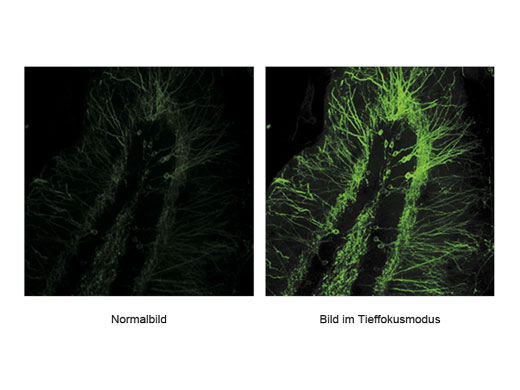
Signalmaximierung im TieffokusmodusIm Tieffokusmodus wird der Durchmesser des Laserstrahls an die Laserstreuung angepasst. Bei In-vivo-Proben mit starker Laserstreuung wird der Strahl verengt, sodass mehr Anregungsphotonen tiefer in die Probe gelangen und hellere Bilder erzeugen. |
|
|
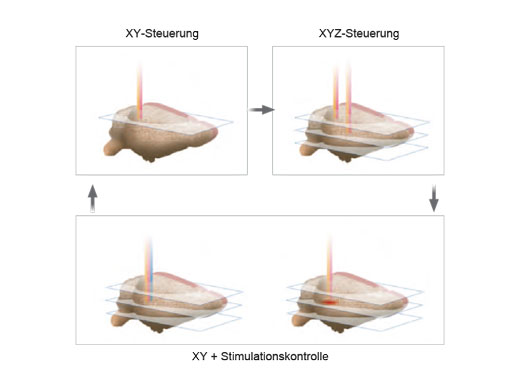
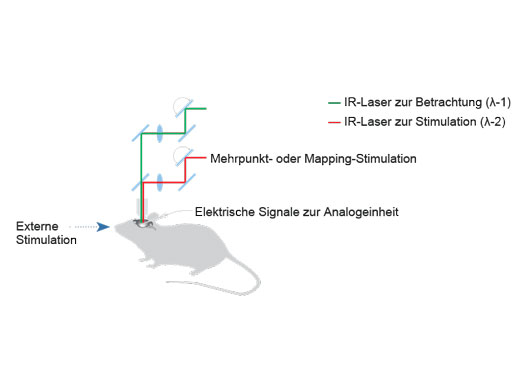
| Unabhängige PhotostimulationskontrolleFolgende Zusatzmodule ermöglichen eine präzise Photostimulation im Mikrosekundenbereich und bei Photobleaching-Experimenten:
Es können beliebige Stimulationsregionen unabhängig von einer definierten Region (ROI) für die Bildgebung definiert werden. Wenn eine höhere Geschwindigkeit erforderlich ist, kann auch eine sequenzielle Random-Access-Mehrpunktstimulation durchgeführt werden. Bei Systemen mit zwei IR-Bildgebungslinien ermöglicht der SIM-Scanner eine Multiphotonenstimulation gleichzeitig mit der Bildgebung. |
|---|
Mikrosekunden-Timing für die Elektrophysiologie und OptogenetikEin Hardware-Sequenzer ermöglicht ein präzises Timing im Mikrosekundenbereich für Stimulations- und Triggering-Ereignisse. Die Stimulation kann räumlich und zeitlich mit dem Bildgebungscan synchronisiert werden, was die Aufnahme einer schnellen Reaktionsdynamik mit präziser Lokalisierung erleichtert. Für die Elektrophysiologie und Optogenetik könnte dies den Unterschied zwischen der Erkennung einer synchronen und einer asynchronen Stimulusreaktion bedeuten. Bei Aufnahmen mit einer Dauer von mindestens zwei Wochen oder Experimenten mit komplexen Verfahren, bei denen zwischen Bildgebungsarten gewechselt werden muss, behält das Sequenzmanager-Softwaremodul die Präzision im Millisekundenbereich bei und liefert auch bei anspruchsvollen In-vivo- und In-vitro-Experimenten hochwertige Daten. Weitere Informationen über multidimensionale Bildgebung und Multi-Area Time-Lapse-Bildgebung |
|
Lösungen
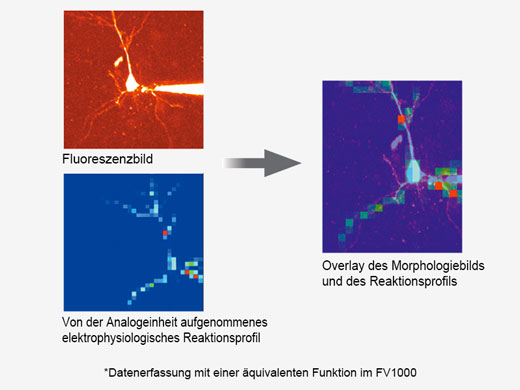
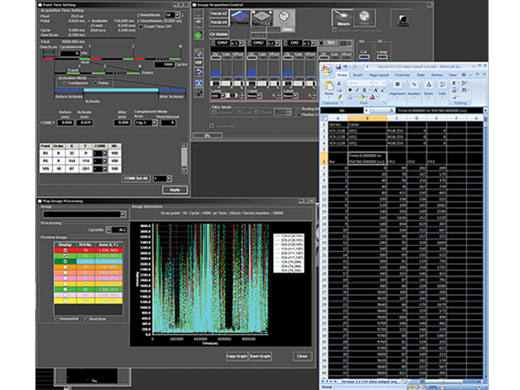
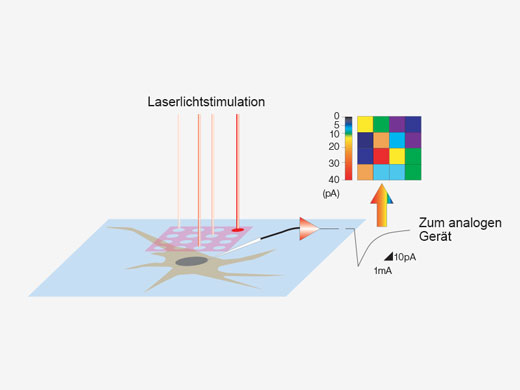
Synchronisation elektrophysiologischer Daten und Laserlichtstimulation mit der AnalogeinheitZur Unterstützung elektrophysiologischer Experimente stehen analoge Eingänge und digitale TTL-E/A zur Verfügung. Die Analogeingangseinheit zeichnet externe Spannungssignale als Bilddaten auf. Mit dem Patch-Clamp-Verfahren gemessene, lichtstimulierte elektrische Signale können mit der Bildaufnahme synchronisiert und als Pseudofarbenintensitäts-Overlay dargestellt werden. Weitere Informationen über die Analogeinheit Die Multipoint Mapping Advanced Software (MMASW) ermöglicht eine präzise Lichtstimulation mehrerer willkürlich ausgewählter Punkte oder von Punkten in einer rechteckigen definierten Region (ROI) bei Mapping-Scans. Sie kann gleichzeitig elektrische Spannungssignale vom Patch-Clamping-System aufzeichnen.
Weitere Informationen über das Softwaremodul für Mehrpunkt- und Mapping-Scans |
|
*Mehrfarbige Punkt-für-Punkt-Lasersteuerung mit einem SIM-Scanner mit mehreren Lasern für gemischte ChR2- und NpHR-Versuche. |
| Erstellung von 3D-Karten von StimulationsreaktionenSpezielle Mapping-Scans verwenden eine Pseudozufallssequenz zur Erstellung präziserer räumlicher Reaktionsprofile bei der Messung der elektrophysiologischen Reaktion auf optische Stimulation. Auf einem hochauflösenden Bild können direkte elektrische Patch-Clamp-Messwerte und Fluoreszenzsignale von Kalzium- oder Spannungsindikatoren überlappend dargestellt werden. Intensitätsschwellenwerte ermöglichen die Definition eines Hochgeschwindigkeits-Mehrpunkt-Scans der aktivsten Regionen, Dieser kann mit einem optionalen Piezo-Z-Laufwerk auf 3D-Reaktionskarten erweitert werden. |
|---|
Ein großes Sehfeld in Kombination mit hoher AuflösungMit der MATL-Funktion (Multiarea Time Lapse) lässt sich die gesamte Probe in hoher Auflösung und in einem breiteren Kontext anzeigen:
|
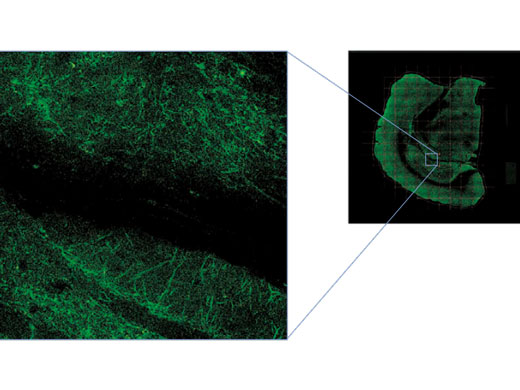
Bilddaten mit freundlicher Genehmigung von Urs Ziegler und Jose Maria Mateos, Center for Microscospy and Image Analysis, Universität Zürich. Die Mauslinie L15 wurde von Pico Caroni, FMI, Basel, zur Verfügung gestellt. |
|---|
Software
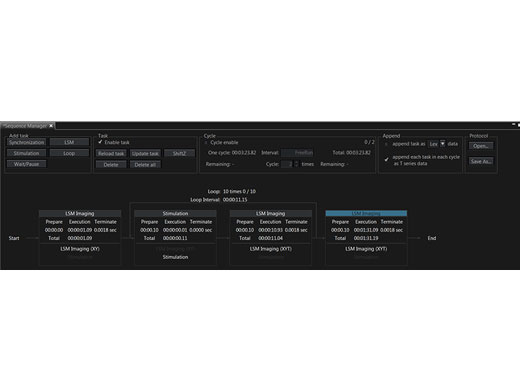
Präzise Steuerung von ExperimentenMit dem Sequenzmanager wird die Koordination von Experimenten zum Kinderspiel. Mithilfe der folgenden Funktionen können komplexe Protokolle mit genauem Timing einfach organisiert und direkt ausgeführt werden:
Zur einheitlichen Ausführung von Experimenten können Protokolle gespeichert und wieder geladen werden. |
|
Trennung überlappender Kanäle durch spektrale DekonvolutionStark überlappende Fluoreszenzspektren können biologische Untersuchungen mit mehreren Markern erschweren. Jetzt können überlappende Spektralkanäle durch spektrale Dekonvolution mit einem Algorithmus zum blinden Entmischen oder durch zuvor gespeicherter Mehrkanalprofile getrennt werden. Das „Übersprechen“ zwischen den Kanälen kann bereits während der Bildaufnahme durch Live-Verarbeitung beseitigt werden. |
Normaler Modus
Die spektrale Überlappung roter und grüner Fluoreszenz verursacht bei der normalen Erfassung eine gegenseitige Störung der Kanäle. | Live-Entmischungsmodus
Im Live-Entmischungsmodus erfolgt bei der Bildaufzeichnung zur besseren Trennung distinkter Fluorophore eine spektrale Dekonvolution. |
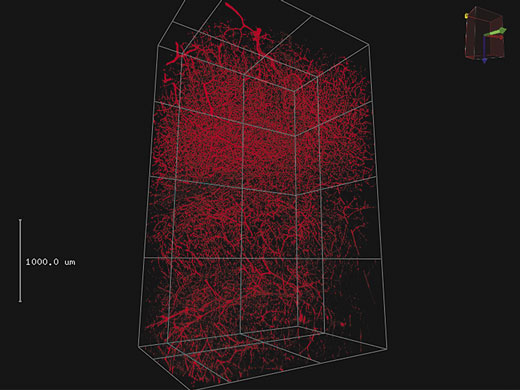
3D-RenderingUmfangreiche Z-Stapel-Daten können zu einer 3D-Darstellung gerendert werden. Für animierte Darstellungen von 3D-Bildern mit Vergrößerung und verschiedenen Kamerawinkeln können wichtige Ansichten als Keyframes gespeichert werden. |
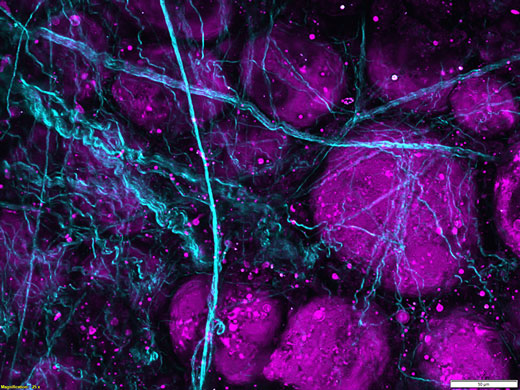
4 mm 3D-Stapel auf mit Texas Red eingefärbtem Blutgefäß im Maushirn.
|
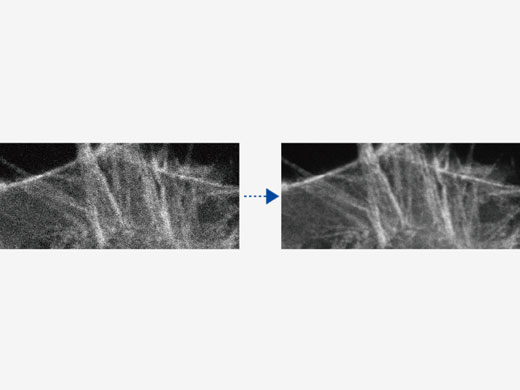
(Links) Rohdaten mit 30 fps, die bei geringer Laserleistung (0,05 %, 488 nm) erfasst wurden.
| Verarbeitung unter Verwendung des gleitenden MittelwertsHigh-Speed-Scanning bei geringer Laserleistung zur Vermeidung von Phototoxizität kann das Signal-Rausch-Verhältnis reduzieren. Die Verarbeitung unter Verwendung des gleitenden Mittelwerts sorgt für die erforderliche Flexibilität zur Anpassung von Hochgeschwindigkeits-Zeitrafferaufnahmen unter Beibehaltung der Zeitskala und der Originaldaten. |
|---|
Makro- und Mikro-BeobachtungDie Verwendung eines Objektivs mit hoher numerischer Apertur (NA), eines motorisierten Tisches und der Olympus-Software ermöglichen Mosaik-Aufnahmen:
|
|
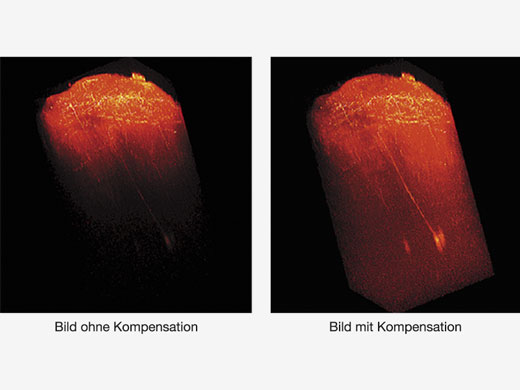
| TiefenhelligkeitskorrekturBei der Betrachtung dicker Proben können Bilder dunkler werden, wenn der Brennpunkt tiefer liegt. Mit der Bright Z Tiefenhelligkeitskorrektur werden Detektorempfindlichkeit und Laserleistung kontinuierlich angepasst, so dass eine gleichmäßige Helligkeit gewährleistet ist. Diese Funktion ergänzt die dynamischen TruResolution-Objektive, die ebenfalls eine automatische Korrektur der sphärischen Aberration je nach Tiefe durchführen. |
|---|
Erweiterte AnalysefunktionenDie Software der FVMPE-RS Bildgebungsplattform ist in die Olympus cellSens Bildanalyse-Software integriert und erweitert die Analysefunktionen des Systems. Optionale Funktionen:
Ebenfalls verfügbar ist die optionale NoviSight 3D-Analysesoftware. Weitere Informationen über die cellSens Bildgebungssoftware |
Spezifikationen
FLUOVIEW FVMPE-RS |
| Einzel-Lasersystem | System mit zwei Laserlinien | Doppellasersystem | ||
|---|---|---|---|---|
| Einheit | Qualifizierte gepulste IR-Laser mit „Negative Chirp“ zur Multiphotonenanregung | Produkte von Spectra-Physics: MAITAI HPDS-OL: 690 nm–1040 nm MAITAI eHPDS-OL: 690 nm–1040 nm INSIGHT X3-OL: 690 nm–1300 nm INSIGHT X3 DUAL/DUALC-OL: 680 nm–1300 nm + 1045 nm Produkte von Coherent: Chameleon Vision I Olympus: 680 nm–1080 nm Chameleon Vision II Olympus: 680 nm–1080 nm Chameleon Vision S Olympus: 690 nm–1050 nm | ||
| Primärer gepulster IR-Laser |
MAITAI HPDS-OL
MAITAI eHPDS-OL INSIGHT X3-OL Chameleon Vision I Olympus Chameleon Vision II Olympus Chameleon Vision S Olympus |
INSIGHT X3 DUAL-OL
INSIGHT X3 DUALC-OL |
MAITAI HPDS-OL
MAITAI eHPDS-OL INSIGHT X3-OL Chameleon Vision I Olympus Chameleon Vision II Olympus Chameleon Vision S Olympus | |
|
Zusätzliche IR-Laserlinie:
Verwendung als zweite Bildgebungslinie/Zweitlaser zur simultanen Stimulation (optionaler SIM-Scanner) | – |
Feste 1045 nm-Linie von
INSIGHT X3 DUAL /DUALC-OL |
MAITAI HPDS-OL
MAITAI eHPDS-OL Chameleon Vision I Olympus Chameleon Vision II Olympus Chameleon Vision S Olympus | |
| Automatische Einfalloptik |
Einfalloptik mit AOM-Abschwächung
(0 %–100 % in Schritten von 0,1 %) Inkl. voll automatisierter Strahlexpander, XY-Shifter und Zwei-Achsen-Winkelausrichtung. (Optik zur automatischen Vierfach-Anpassung von 4 Achsen) Direktkopplung mit dem Lasereingang der Scanning-Einheit. |
Einfalloptik mit AOM-Abschwächung in 2 Sets (0 %–100 % in Schritten von 0,1 %)
Inkl. 2 Sets mit voll automatisiertem Strahlexpander, XY-Shifter und Zwei-Achsen-Winkelausrichtung. (Optik zur automatischen Vierfach-Anpassung von 4 Achsen) Direktkopplung mit dem Lasereingang der Scanning-Einheit. | ||
| Optik zur IR-Laser-Kombination | – | Motorisierter Lichtweg-Umschalter mit DM900, DM1000R, DM1100 zur Kombination von zwei IR-Wellenlängen für die Bildgebung | ||
| Optionaler Laser im sichtbaren Bereich zur Stimulation | 405 nm/50 mW, 458 nm/20 mW, 588 nm/20 mW Laserquelle mit AOTF-Abschwächung. 0 %–100 % in Schritten von 0,1 %, < 2 μs Anstiegszeit | |||
| Scanning-Einheit | Scan-Methode | Lichtablenkung über 2 silberbeschichtete Galvanometer-Scan-Spiegel oder einen silberbeschichteten Resonanz-Scan-Spiegel | ||
| Scanning-Geschwindigkeit |
Galvanometer-Scanner (normale Bildgebung): 512 × 512 mit 1,1 s–264 s, Pixeltakt: 2 μs–1000 μs
Resonanz-Scanner (Hochgeschwindigkeitsbildgebung): 30 fps bei 512 × 512, 438 fps bei 512 × 32 | |||
| Scan-Modus | XY, XYZ, XYT, XYZT, Freihandlinie, XZ, XT, XZT, PointT | |||
|
Galvanometer-Scanner
(Normale Bildgebung) |
Galvanometer-ROI-Scanning: Rechteck-Clip, Ellipse, Polygon, Freihandfläche, Linie, Freihandlinie und Punkt
Zoom: 1,0x–50,0x in Schritten von 0,01x, unterstützt Rotation und Schwenken um 0°–360° Scanfeldzahl: 18 Bildgröße: 64 × 64–4096 × 4096 | |||
|
Resonanz-Scanner
(Hochgeschwindigkeitsbildgebung) |
Resonanz-ROI-Scannen, Rechteck-Clip, Linie
Zoom: 1,0x–8,0x in Schritten von 0,01x Scanfeldzahl: 18 Bildgröße: 512 × 512 | |||
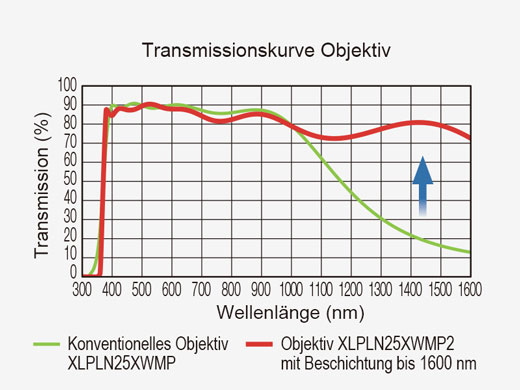
| Optische Beschichtung | IR-Support-Optik mit 1600er Beschichtung | |||
| Non-Descanned MPE Bildgebungsdetektoren |
Auflichtdetektion: 2- oder 4-Kanalkonfiguration: 2-PMT-Konfiguration, 4-PMT-Konfiguration oder Konfiguration mit 2 PMT + 2 gekühlten GaAsP-PMTs
Durchlichtdetektion: Einheit mit 2 PMTs und Kondensator mit hoher NA | |||
| Durchlicht-Detektor | Modul mit integriertem externem Durchlicht-Photomultiplier-Detektor und 100-W-Halogenlampe, motorisiertem Wechsel, faseroptischer Adapter für Mikroskopstativ | |||
| Z-Antrieb |
Integriertes motorisches Fokusmodul des Mikroskops, kleinster Schritt 0,01 μm
Optional: stabiler Piezo-Objektivrevolver*1 | |||
| Optionaler Scanner zur simultanen Stimulation |
Scanner zur hochsynchronen simultanen Stimulation, einschließlich eines Sets aus Galvanometer-Scanner, VIS- und IR-Lasereingang
ROI-Scanning: Rechteck-Clip, Ellipse, Polygon, Tornado, Freihandfläche, Linie, Freihandlinie und Punkt. | |||
| Optionale Analog- und Digital-E/A-Box | 4-Kanal-Analogsignal-Eingang, digitaler 6-Kanal-TTL-Trigger-Eingang, digitaler 5-Kanal-TTL-Trigger-Ausgang. Scanner-Timing-Ausgang | |||
| Betriebsumgebung | Raumtemperatur: 20 °C–25 °C, Feuchtigkeit: 75 % oder weniger bei 25 °C, erfordert kontinuierliche (24-stündige) Stromversorgung | |||
| Größe des erschütterungssicheren Tisches |
1500 mm × 1650* mm
*1800 mm bei dem Invers-Mikroskopsystem |
1500 mm × 1650* mm
*1800 mm bei dem Invers-Mikroskopsystem | 1500 mm × 2000 mm | |
| Software | Grundlegende Funktion |
Dunkelkammer-Design der GUI. Vom Anwender veränderbares Layout.
Ladefunktionen für Erfassungsparameter. Festplattenaufzeichnung möglich, Anpassung der Laserleistung und HV mit Z-Stapelerfassung. Z-Stapel mit Alpha-Blending, Projektion mit maximaler Helligkeit, ISO-Oberflächen-Rendering | ||
| IR-Lasersteuerung | Voll integrierte IR-Laser-Wellenlängensteuerung und Tieffokusmodus | |||
| Optionale Software für den motorisierten XY-Tisch |
Motorisierte XY-Tischsteuerung Profilbildaufnahme zur einfachen Lokalisierung von Zielobjekten. Kachelerfassung und Software-Bildstiching.
Definition mehrerer Bereiche für Zeitrafferaufnahmen. | |||
| Optionale Software für Mapping und Mehrpunktstimulation. |
Software für Mehrpunktstimulation und Datenerfassung. Mapping-Mehrpunktstimulation zur Erstellung eines Reaktionsprofils.
Filterfunktion zur Auswahl von Punkten. Mehrpunktstimulation. Einzel- oder Mehrfachstimulation. Unabhängige Auswahl der Stimulationswellenlänge für jeden Punkt. | |||
|
Optionaler Sequenz-
Manager |
Erweiterte programmierbare Software zum Definieren mehrerer Bildgebungs-/Stimulationsvorgänge und zu deren Ausführung durch einen Hardware-Sequenzer.
Mindestverzögerung von 100 ms zwischen den Vorgängen. | |||
| Optionale Autokompensationssoftware |
Software zur automatischen Kompensation der sphärischen Aberration.
Steuerung der Objektivlinse mit der Funktion zur automatischen Kompensation der sphärischen Aberration. Automatische Anpassung des motorisierten Korrekturrings, um die beste Position bei einer bestimmten Beobachtungstiefe zu finden. Automatische Anpassung des Korrekturrings bei der Z-Bewegung. | |||
*1 Nicht überall erhältlich. |
TruResolution Objektive |
| FV30-AC10SV | FV30-AC25W | |
|---|---|---|
| Vergrößerungen | 10 | 25 |
| Nummerische Apertur (NA) | 0,6 | 1,05 |
| AA | 8 mm | 2 mm |
| Deckglasdicke | 0 mm – 0,23 mm | 0 mm – 0,23 mm |
| Immersionsflüssigkeit | SCALEVIEW-A2 (für Wasser, Silikonöl und normales Öl) | Wasser |
| Besondere Eigenschaften | Autokompensation, optimiert für Multiphotonen-Bildgebung | Autokompensation, optimiert für Multiphotonen-Bildgebung |
| Abmessungen (B × T x H) | 56 mm × 106,5 × 95 mm | 56 mm × 106,5 × 101 mm |
| Gewicht | Etwa 1 kg | Etwa 1 kg |