Selective Isolation of Adherent Single Cells
Sandra Lubos1,2, Nils Körber1, Heide Marie Resch1, Iris Augustin2, Stefan Niehren1
1Molecular Machines & Industries GmbH, Eching, Germany
2University of Applied Sciences, Weihenstephan-Triesdorf, Germany
November 15, 2017
Abstract
The isolation of single cells is an essential prerequisite for many research projects in pathology, oncology, and forensics.
The MMI CellEctor is designed to facilitate selective isolation of single cells in suspensions. This study illustrates the development of the novel “Shake Mode” feature for the efficient uptake of adherent cells. The unique module has been integrated into the MMI CellTools software to promote gentle detachment of adherent cells. Thus, the Shake Mode enables efficient transfer of cells in suspension as well as living adherent cells.

Figure 1: MMI CellEctor system on the Olympus IX83 inverted microscope. This system is also equipped with the MMI CellCut laser microdissection system to isolate cells in tissue.
Introduction
There are many reasons why it is important to study single cells rather than doing bulk analysis. In oncology, different mutations of tumor suppressor genes or oncogenes in circulating tumor cells (CTCs) or in metastases are sought to be identified, since these sub-tumor species are a key factor contributing to patients’ survival and may react differently to therapy than the primary tumor. In forensics, DNA profiles are studied to assign samples at a crime scene to the victim and
perpetrator.
Even though a cell population may have the same DNA, individual cells often vary in their expression profile. Therefore, many researchers are interested in the RNA and protein levels of cells in different tissues or under different conditions such as time, stress, toxins, growth factors, etc.
Many methods to isolate single cells have been developed, both for tissue sections and for cells in suspension. However, most of these methods lack the ability to select individual cells, a common example of which is fluorescence-activated cell sorting (FACS). The MMI CellEctor is a proven tool to selectively isolate single cells in solution1-7. The instrument consists of a microscope and a pump connected to a software-controlled micro-capillary. This allows researchers to
optically select a single cell that is then taken up by the micro-capillary with a defined volume. The pump volume can be adjusted to generate the forces required for cell aspiration and dispensing. However, it has been difficult to aspirate living adherent cells since the adhesive forces prevent detachment and uptake.
Living adherent cells can typically be detached by trypsinization, but the cells’ RNA and protein expression profile will be altered by this treatment, resulting in inaccurate transcriptomics or proteomics data.
In this application note, we establish optimal conditions for the isolation of single adherent cells, avoiding trypsinization while making sure that the isolated cells are viable for further cultivation and downstream analysis. We adjusted and tested the parameters to selectively and sensitively aspire one cell without any contamination by additional cells and effectively deposited this cell.
Material and Methods
HeLa cells were cultivated in Dulbecco’s Modified Eagle’s medium (DMEM), supplemented with FBS, nonessential amino acids, L-Glutamin, and Penicillin-Streptomycin at 37 °C (98.6 °F) and 10% CO2. 300 μl of a cell suspension with 1 × 104 cells/ml were transferred into an 8‑well slide (glass bottom or treated polyethylene, ibidi) and incubated overnight.
Single cells were selected, aspired, and deposited using the MMI CellEctor mounted on an Olympus IX83 inverted microscope. The MMI CellEctor was equipped with an 80 µm capillary that was prefilled with cell culture medium. The capillary was rinsed with MMI Capillary Clean and cell culture medium after 10 steps of cell acquisition and deposition. Cells were deposited into a selected well on a second 8‑well slide, which has been prepared with 300 µl cell
culture medium and pre-incubated at 37 °C (98.6 °F), 10% CO2.
Results
To identify optimal conditions for the selective isolation of single HeLa cells and their transfer to a separate slide, we varied different parameters, such as pump volume and speed, dwell time, and the micro-capillary angle. However, the flow force generated by the pump turned out to be insufficient to detach adherent cells. Since the microscope stage can be precisely moved using the MMI CellTools software, we slightly rotated the stage to push the selected cell toward the capillary walls (Figure 2).
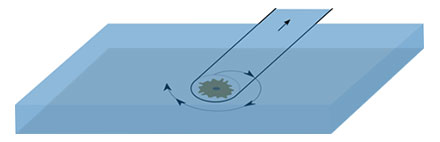
Figure 2: Schematic representation of the Shake Mode.
This gentle mechanical force was sufficient to detach the cell, enabling subsequent aspiration by the micro-capillary. This led to the development of Shake Mode as a novel software feature within the MMI CellTools package. The Shake Mode can be customized by the user to optimize its rotation dimensions (defined by the x- and y-distances from the rotation center), the rotation speed, and the number of repetitions. In this example, the Shake Mode was applied with a radius of 60 µm (x = y = 60 µm) at a speed of 100 µm/s and 2 repetitions. These conditions allowed for effective HeLa cell detachment and successful aspiration (Figure 3).
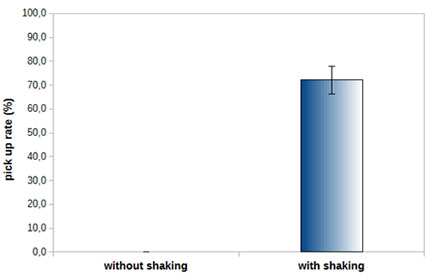
Figure 3: Pick up rates of HeLa Cells with and without using the Shake Mode. Adherent single cells can be aspirated with more than a 70% success rate using the Shake Mode. Using the same conditions but not applying the Shake Mode, cells cannot be aspirated at all.
Once we applied the Shake Mode, we found that an aspiration volume of 50 nl and a deposition volume of 60 nl (both at 6 nl/s) led to effective, reproducible cell transfer conditions. We adjusted the capillary angle to 90°. In addition, the capillary was allowed to dwell for 3 seconds after each cell deposition cycle. A typical cycle of single cell aspiration and deposition is depicted in Figure 4.
|
|
|
|
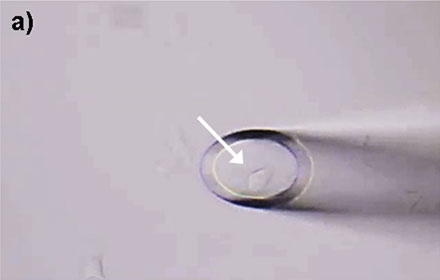
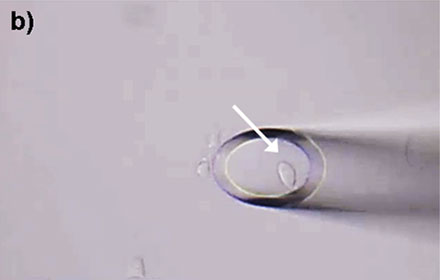
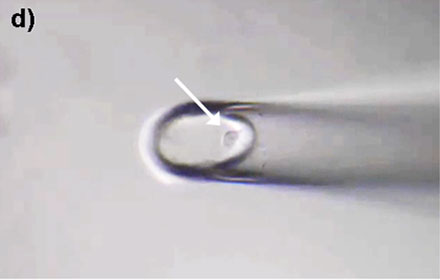
Figure 4: HeLa cells* can be selectively isolated by the MMI CellEctor. a) A single cell is chosen and approached by the micro-capillary. b) Using the Shake Mode, the microscope stage rotates and pushes the cell towards the micro-capillary, which causes the cell to detach from the cell chamber. The cell can then selectively be aspirated. c) The aspirated cell does not affect surrounding cells. d) The cell is deposited in the target well. Check out the video or scan the QR code below.
The transfer procedure is very gentle, enabling HeLa cells to stay vital. After the transfer into a new cell culture dish, the cells were able to grow and divide, forming the basis for a new culture (Figure 5) 9.
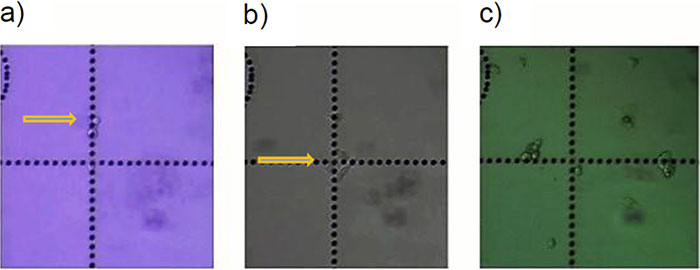
Figure 5: HeLa cells* in cell culture (a) 1 day, (b) 2 days, and (c) 5 days after transfer with the MMI CellEctor.
Discussion
Applying the novel MMI CellTools Shake Mode software feature, this study demonstrates that a single cell is taken up by a micro-capillary without aspiration of additional, unwanted cells. In addition, the conditions were optimized to efficiently release aspirated cells at the target position.
For different cells types, the protocol might need some adjustment since the cells may have different properties, such as adhesion, size, and shape. However, the parameters described above can be applied as a starting point for subsequent fine tuning.
This work reveals that, using the novel Shake Mode, adherent cells can be detached from surfaces without compromising their viability or integrity. Thus, single cells can now be selectively isolated and utilized for downstream applications, even for sensitive RNA expression analysis.
Application note posted with permission from Molecular Machines and Industries.
References
- Pizon, M. et al. Heterogeneity of circulating epithelial tumour cells from individual patients with respect to expression profiles and clonal growth (sphere formation) in breast cancer. Ecancermedicalscience 7, (2013).
- Zimon, D., Pizon, M., Stein, E.-L., Pachmann, U. A. & Pachmann, K. Vemurafenib to eliminate BRAF-mutated circulating epithelial tumor cells (CETCs) from blood of patients with malignant melanoma. J. Clin. Oncol. 33, 11031 (2015).
- Hjortland, G. O. et al. Genome wide single cell analysis of chemotherapy resistant metastatic cells in a case of gastroesophageal adenocarcinoma. BMC Cancer 11, 455 (2011).
- Lapin, M. et al. MINDEC-An Enhanced Negative Depletion Strategy for Circulating Tumour Cell Enrichment. Scientific Reports 6, 28929 (2016).
- Lapin, M. et al. Single-cell mRNA profiling reveals transcriptional heterogeneity among pancreatic circulating tumour cells. BMC Cancer 17, 390 (2017).
- Alberter, B., Klein, C. A. & Polzer, B. Single-cell analysis of CTCs with diagnostic precision: opportunities and challenges for personalized medicine. Expert Rev. Mol. Diagn. 16, 25–38 (2016).
- Sukhbaatar, N. et al. Two different, mutually exclusively distributed, TP53 mutations in ovarian and peritoneal tumor tissues of a serous ovarian cancer patient: indicative for tumor origin? Mol. Case Stud. (2017). doi:10.1101/mcs.a001461
- Huang, H.-L. et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 17, 36 (2010).
- Lubos, S. Verfahrensoptimierung der kapillarbasierten Einzelzellisolation von lebenden adhärenten Zellen. (Hochschule Weihenstephan-Triesdorf, 2017).
*Although it became one of the most important cell lines in medical research, it’s imperative that we recognize Henrietta Lacks’ contribution to science happened without her consent. This injustice, while leading to key discoveries in immunology, infectious disease, and cancer, also raised important conversations about privacy, ethics, and consent in medicine.
To learn more about the life of Henrietta Lacks and her contribution to modern medicine, click here.
http://henriettalacksfoundation.org/
Productos usados para esta aplicación
se ha añadido correctamente a sus marcadores
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.