Visible light is a complex phenomenon that is classically explained with a simple model based on propagating rays and wavefronts, a concept first proposed in the late 1600s by Dutch physicist Christiaan Huygens. Electromagnetic radiation, the larger family of wave-like phenomena to which visible light belongs (also known as radiant energy), is the primary vehicle transporting energy through the vast reaches of the universe. The mechanisms by which visible light is emitted or absorbed by substances, and how it predictably reacts under varying conditions as it travels through space and the atmosphere, form the basis of the existence of color in our universe.
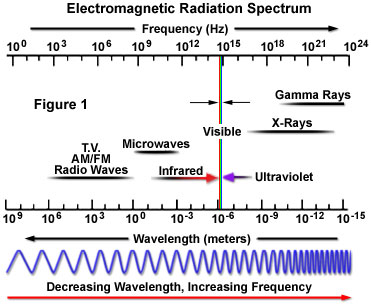
The term electromagnetic radiation, coined by Sir James Clerk Maxwell, is derived from the characteristic electric and magnetic properties common to all forms of this wave-like energy, as manifested by the generation of both electrical and magnetic oscillating fields as the waves propagate through space. Visible light represents only a small portion of the entire spectrum of electromagnetic radiation (as categorized in Figure 1), which extends from high-frequency cosmic and gamma rays through X-rays, ultraviolet light, infrared radiation, and microwaves, down to very low frequency long-wavelength radio waves.
The link between light, electricity, and magnetism was not immediately obvious to early scientists who were experimenting with the fundamental properties of light and matter. Infrared light, which lies beyond the longer red wavelengths of visible light, was the first "invisible" form of electromagnetic radiation to be discovered. British scientist and astronomer William Herschel was investigating the association between heat and light with a thermometer and a prism when he found that the temperature was highest in the region just beyond the red portion of the visible light spectrum. Herschel suggested that there must be another type of light in this region that is not visible to the naked eye.
Ultraviolet radiation, at the other end of the visible spectrum, was discovered by Wilhelm Ritter, who was one of the first scientists to investigate the energy associated with visible light. By observing the rate at which various colors of light stimulate darkening of paper saturated with a solution of silver nitrate, Ritter discovered that another invisible form of light, beyond the blue end of the spectrum, yielded the fastest rates.
Electromagnetic Wave Propagation
Explore wave propagation through space with a sinusoidal representation of electromagnetic radiation. Interact with the tutorial to vary the wavelength and the fill factor of electric and magnetic field vectors.
Start Tutorial »Electricity and magnetism were first associated in 1820 when Danish physicist Hans Christian Oersted discovered that electrical current flowing through a wire could produce deflections of a compass needle. Later that same year, French scientist André-Marie Ampère demonstrated that two wires carrying electrical currents could be made to attract or repel each other, in a fashion similar to that of magnetic poles. During the next few decades, additional investigations following these leads produced an increasing amount of evidence that electricity and magnetism were very closely related to each other.
Finally, in 1865, Scottish scientist James Clerk Maxwell expanded his kinetic theory of gases to mathematically explain the links between electricity and magnetism. Maxwell speculated that the two phenomena were so closely bound that they often acted together as electromagnetism, and discovered that alternating current would produce waves composed of both entities that radiated out into space at the speed of light. From these observations, he concluded that visible light was a form of electromagnetic radiation.
An electromagnetic wave travels or propagates in a direction that is oriented at right angles to the vibrations of both the electric (E) and magnetic (B) oscillating field vectors, transporting energy from the radiation source to an undetermined final destination. The two oscillating energy fields are mutually perpendicular (illustrated in Figure 2) and vibrate in phase following the mathematical form of a sine wave. Electric and magnetic field vectors are not only perpendicular to each other, but are also perpendicular to the direction of wave propagation. By convention, and to simplify illustrations, the vectors representing the electric and magnetic oscillating fields of electromagnetic waves are often omitted, although they are understood to still exist.
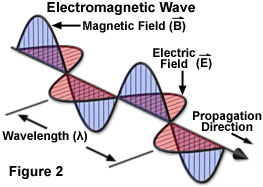
Whether taking the form of a signal transmitted to a radio from the broadcast station, heat radiating from a fireplace, the dentist's X-rays producing images of teeth, or the visible and ultraviolet light emanating from the sun, the various categories of electromagnetic radiation all share identical and fundamental wave-like properties. Every category of electromagnetic radiation, including visible light, oscillates in a periodic fashion with peaks and valleys (or troughs), and displays a characteristic amplitude, wavelength, and frequency that together define the direction, energy, and intensity of the radiation.
The classical schematic diagram of an electromagnetic wave presented in Figure 2 illustrates the sinusoidal nature of oscillating electric and magnetic component vectors as they propagate through space. As a matter of convenience, most illustrations depicting electromagnetic radiation purposely omit the magnetic component, instead representing only the electric field vector as a sine wave in a two-dimensional graphical plot having defined x and y coordinates. By convention, the y component of the sine wave indicates the amplitude of the electric (or magnetic field), while the x component represents time, the distance traveled, or the phase relationship with another sine wave.
A standard measure of all electromagnetic radiation is the magnitude of the wavelength (in a vacuum), which is usually stated in units of nanometers (one-thousandth of a micrometer) for the visible light portion of the spectrum. The wavelength is defined as the distance between two successive peaks (or valleys) of the waveform (see Figure 2). The corresponding frequency of the radiated wave, which is the number of sinusoidal cycles (oscillations or complete wavelengths) that pass a given point per second, is proportional to the reciprocal of the wavelength. Thus, longer wavelengths correspond to lower frequency radiation and shorter wavelengths correspond to higher frequency radiation. Frequency is usually expressed in quantities of hertz (Hz) or cycles per second (cps).
The hertz was designated as a standard unit of electromagnetic radiation frequency in recognition of the work of German physicist Heinrich Hertz, who succeeded in generating and performing experiments with electromagnetic waves in 1887, eight years after the death of Maxwell. Hertz produced, detected, and even measured the wavelength (approximately one meter) of radiation that is now classified in the radiofrequency range. David Hughes, a London-born scientist who was a music professor in his early career, may have actually been the first investigator to succeed in the transmission of radio waves (in 1879), but after failing to convince the Royal Society, he decided not to publish his work, and it wasn't recognized until many years later.
Basic Electromagnetic Wave Properties
Examine how the frequency, energy, and wavelength of an electromagnetic wave are related, and how changing one automatically produces a corresponding change in the other two (and the color of visible light).
Start Tutorial »The different wavelengths and frequencies comprising the various forms of electromagnetic radiation are fundamentally similar in that they all travel at the same speed—about 186,000 miles per second (or approximately 300,000 kilometers per second), a velocity commonly known as the speed of light (and designated by the symbol c). Electromagnetic radiation (including visible light) travels 149 million kilometers (93 million miles) from the sun to Earth in about 8 minutes. In contrast, an automobile speeding at 100 kilometers per hour (60 miles per hour) would require 177 years to make the same one-way trip. In only one second, light can circumnavigate the Earth seven times.
The wavelength of light, and all other forms of electromagnetic radiation, is related to the frequency by a relatively simple equation:
where c is the speed of light (in meters per second), ν is the frequency of the light in hertz (Hz), and λ is the wavelength of the light measured in meters. From this relationship one can conclude that the wavelength of light is inversely proportional to frequency. An increase in frequency produces a proportional decrease in the wavelength of light, with a corresponding increase in the energy of photons that comprise the light. Upon entering a new medium (such as glass or water from air), the speed and wavelength of light is reduced, although the frequency remains unaltered.
Under normal circumstances, when traveling through a uniform medium, such as air or a vacuum, light propagates in straight lines until interaction with another medium or material induces a path change, through refraction (bending) or reflection. The intensity may be also reduced as a result of absorption by the medium. If the light waves pass through a narrow slit or aperture (hole), then they can be diffracted or dispersed (scattered) to form a characteristic diffraction pattern. In accordance with the well-known inverse square law, the intensity (or irradiance) of electromagnetic radiation is inversely proportional to the square of the distance traveled. Thus, after light has traveled twice a given distance, the intensity drops by a factor of four.
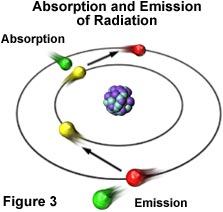
Visible light displays classical wave-like properties, but it also exhibits properties reminiscent of particles, which are manifested through entities that possess energy and momentum (but no mass), and are referred to as photons. The atom is the source of all forms of electromagnetic radiation, whether visible or invisible. Higher-energy forms of radiation, such as gamma waves and X-rays, are produced by events that occur to disrupt the nuclear stability of the atom. Radiation having lower energy, such as ultraviolet, visible, and infrared light, as well as radio and microwaves, originate from the electron clouds that surround the nucleus or the interaction of one atom with another. These forms of radiation occur due to fact that electrons moving in orbits around the nucleus of an atom are arranged in different energy levels within their probability distribution functions. Many of the electrons can absorb additional energy from external sources of electromagnetic radiation (see Figure 3), which results in their promotion to an inherently unstable higher energy level.
Eventually, the "excited" electron loses the extra energy by emitting electromagnetic radiation of lower energy and, in doing so, falls back into its original and stable energy level. The energy of the emitted radiation equals the energy that was originally absorbed by the electron minus other small quantities of energy lost through a number of secondary processes.
Electromagnetic radiation energy levels can vary to a significant degree depending upon the energy of source electrons or nuclei. For example, radio waves possess significantly less energy than do microwaves, infrared rays, or visible light, and all of these waves contain far less energy than ultraviolet light, X-rays, and gamma waves. As a rule, higher electromagnetic radiation energies are associated with shorter wavelengths than similar forms of radiation having lower energy. The relationship between the energy of an electromagnetic wave and its frequency is expressed by the equation:
where E is the energy in kilojoules per mole, h is Planck's constant, and the other variables are defined as discussed previously. Based on this equation, the energy of an electromagnetic wave is directly proportional to its frequency and inversely proportional to the wavelength. Thus, as frequency increases (with a corresponding decrease in wavelength), the electromagnetic wave energy increases, and vice versa. Selected characteristics of the different types of electromagnetic radiation, as defined by their wavelength, frequency, and energy levels, will be reviewed individually in the following paragraphs.
Even though electromagnetic radiation is customarily described by the wavelength and frequency of the waveforms, other characteristic properties are important when considering how waves propagate through space. Presented in Figure 4 are various waveforms representing common states that are utilized to describe the degree of uniformity of electromagnetic radiation. Because visible light is the most commonly discussed form of radiation, the examples illustrated in Figure 4 are representative of wavelengths in this spectral region. For example, monochromatic light consists of waves all having the same wavelength and frequency, or macroscopically, the same color in visible light. In contrast, polychromatic visible light usually appears as white due to contributions from the mixture of all or most wavelengths in the spectrum ranging between 400 and 700 nanometers.
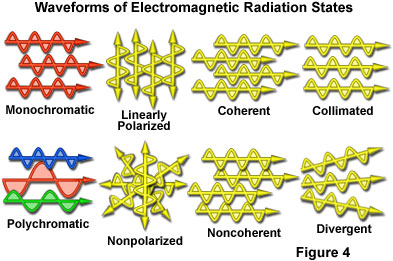
When light is non-polarized (Figure 4), the electric field vectors vibrate in all planes lying perpendicular to the direction of propagation. Light that has been reflected from a smooth surface at a critical angle, or passed through polarizing filters, assumes a plane-polarized orientation with all of the electric vectors vibrating in a single plane perpendicular to the direction of propagation. Light from the sun, and a majority of the common incandescent and fluorescent visible light sources, is non-polarized, while light seen through polarizing lenses of custom sunglasses is polarized in the vertical direction. In some instances, light can be elliptically or circularly polarized when it passes through materials that have more than one refractive index (birefringent or doubly refracting substances).
Most artificial and natural light sources emit non-coherent light that displays a variety of phase relationships among the wavelengths present in the spectrum (Figure 4). In this case, the peaks and valleys of the vibrational states in individual waves do not coincide in either space or time. Coherent light is composed of wavelengths that are in phase with each other, and behaves in a very different manner than non-coherent light with respect to the optical properties and interaction with matter. Wavefronts produced by coherent light have electric and magnetic vector vibrations that oscillate in phase, have low divergence angles, and are usually composed of monochromatic light or wavelengths that have a narrow distribution. Lasers are a common source of coherent light.
Light waves that have coaxial, relatively non-diverging paths as they travel through space are termed collimated. This organized form of light does not spread or converge to a significant degree over comparatively long distances. Collimated light forms a very tight beam, but does not necessarily have a narrow band of wavelengths (nor must it be monochromatic), a common phase relationship, or a defined state of polarization. Wavefronts of collimated light are planar and perpendicular to the axis of propagation. In contrast, divergent or non-collimated light spreads to varying degrees while traveling through space, and must be passed through a lens or aperture in order to be collimated or focused.
Gamma rays - High-energy radiation that possesses the highest frequency (and shortest wavelengths), gamma rays are emitted as the result of transitions within the atomic nucleus, including nuclei of certain radioactive materials (natural and artificial). Gamma waves also originate from nuclear explosions and a variety of other sources in outer space. These powerful rays possess tremendous penetrating ability and have been reported to be able to pass through three meters of concrete! Individual gamma-ray photons contain so much energy that they are easily detected, but the extremely small wavelength limits the experimental observation of any wavelike properties. Gamma rays originating from the hottest regions of the universe, including supernova explosions, neutron stars, pulsars, and black holes, travel through vast distances in space to reach the Earth. This high-energy form of radiation has wavelengths less than one-hundredth of a nanometer (10 picometers), photon energies greater than 500 kiloelectron-volts (keV), and frequencies exceeding 30 exahertz (EHz).
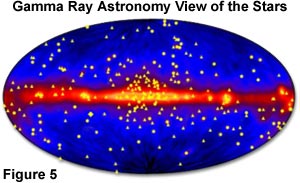
Exposure to gamma rays can induce mutations, chromosome aberrations, and even cell death, as is often observed in some forms of radiation poisoning. However, by controlling the emission of gamma rays, radiologists can re-direct the high energy levels to combat disease and help cure some forms of cancer. Gamma ray astronomy is a relatively new science that collects these high-energy waves in order to produce images of the universe, as illustrated in Figure 5. This technique affords scientists opportunities to observe distant celestial phenomena in the search for new physical concepts, and to test theories that cannot be challenged by experiments performed here on the Earth.
X-rays - Electromagnetic radiation having a frequency just above the ultraviolet (but below the gamma) range is classified as X-rays, and is energetic enough to pass easily through many materials, including the soft tissues of animals. The high penetration depths of these powerful waves, coupled with their ability to expose photographic emulsions, has led to the extensive use of X-rays in medicine to investigate textures in the human body, and in some cases, as a therapeutic or surgical tool. In the same manner as higher-energy gamma rays, uncontrolled exposure to X-rays can lead to mutations, chromosome aberrations, and other forms of cell damage. Traditional radiographic imaging methods essentially produce nothing more than shadow castings of dense material, rather than detailed images. Recent advances in X-ray focusing technique using mirror optics, however, has led to significantly more detailed imagery from a variety of objects utilizing X-ray telescopes, X-ray microscopes, and interferometers.
Hot gases in outer space emit a powerful spectrum of X-rays, which are utilized by astronomers to gain information about the origin and characteristics of interstellar regions of the universe. Many extremely hot celestial objects, including the sun, black holes, and pulsars, emit primarily in the X-ray region of the spectrum and are the subjects of astronomical X-ray investigations. The frequency spectrum of X-rays spans a very large range with the shortest wavelengths approaching the diameter of an atom. However, the entire X-ray spectral region traverses the length scale between approximately 10 nanometers and 10 picometers. This wavelength range makes X-ray radiation an important tool to geologists and chemists for characterizing the structure and properties of crystalline materials, which have periodic structural features on a length scale comparable to the X-ray wavelengths.
Ultraviolet Light - Often abbreviated (uv), ultraviolet radiation propagates at frequencies just above those of violet in the visible light spectrum. Although the low-energy end of this spectral region is adjacent to visible light, ultraviolet rays at the upper end of their frequency range have enough energy to kill living cells and produce significant tissue damage. The sun is a constant source of ultraviolet radiation, but the atmosphere of the Earth (primarily ozone molecules) effectively blocks a majority of the shorter wavelengths of this potentially lethal radiation stream, thus affording a suitable living environment for plants and animals. Photon energies in the ultraviolet are sufficient to ionize the atoms from a number of gas molecules in the atmosphere, and this is the process by which the ionosphere is created and sustained. Although small doses of this relatively high-energy light can promote the production of vitamin D in the body, and cause minimal tanning of the skin, too much ultraviolet radiation can lead to serious sunburn, permanent retinal damage, and the promotion of skin cancer.
Ultraviolet light is utilized extensively in scientific instruments to probe the properties of various chemical and biological systems, and it is also important in astronomical observations of the solar system, galaxy, and other parts of the universe. Stars and other hot celestial objects are strong emitters of ultraviolet radiation. The ultraviolet wavelength spectrum ranges from about 10 to approximately 400 nanometers, with photon energies ranging between 3.2 and 100 electron-volts (eV). This category of radiation has applications in water and food treatment as an anti-microbial agent, as a photocatalyst for caged compounds, and is utilized to harden casts in medical treatments. The germicidal activity of ultraviolet light occurs at wavelengths less than approximately 290 nanometers. A market for blocking and filtering compounds employed in skin lotions, sunglasses, and window tints aims at controlling exposure to ultraviolet light from the sun.
Some insects (notably honeybees) and birds have sufficient visual sensitivity in the ultraviolet region to respond to longer wavelengths, and may rely upon this capability in navigation. Humans are limited in their sensitivity to ultraviolet radiation due to absorption by the cornea of shorter wavelengths, and by strong absorption in the eye lens at wavelengths longer than 300 nanometers.
Visible light - The rainbow of colors associated with the visible light spectrum represents only about 2.5 percent of the entire electromagnetic spectrum, and includes photons with energies between approximately 1.6 to 3.2 electron-volts. Color is not a property of the light itself, but the perception of color occurs through the combined response of the human eye-nerve-brain sensing system. The visible region of the electromagnetic spectrum lies within a narrow frequency band, from approximately 384 to 769 terahertz (THz) and is perceived as colors ranging from deep red (wavelength of 780 nanometers) to deep violet (400 nanometers).
The low-energy, long-wavelength red colors (622-780 nanometers) are followed in sequence by orange (597-622 nanometers), yellow (577-597 nanometers), green (492-577 nanometers), blue (455-492 nanometers), and finally, relatively high-energy, short-wavelength violet (455 nanometers and below). An easy method to remember the order (in increasing frequency) of the colors in the visible light spectrum is with the mnemonic acronym ROY G BIV (Red, Orange, Yellow, Green, Blue, Indigo, Violet,), as taught to millions of school children for nearly a century (although indigo is no longer considered a pertinent color by some scientists).
Division of the visible light spectrum into color regions based on physical properties is straightforward, but the manner in which color is sensed is not as obvious. Perception of color results from subjective responses of the human sensing system to the various frequency regions of the visible spectrum, and a variety of different combinations of light frequencies can produce the same visual response of "seeing" a specific color. Humans may perceive the color green, for example, in response to a combination of light of several colors, none of which are necessarily composed of "green" wavelengths.
Visible light is the basis for all life on Earth, and is captured by primary producers or autotrophs, such as green plants. These fundamental members of the biological food chain harness sunlight as the source of energy for manufacturing their own food and biochemical building blocks. In return, autotrophs release oxygen, upon which all animals depend, as a by-product.
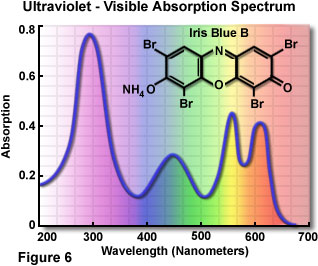
In 1672, Sir Isaac Newton studied the interaction of visible light with a glass prism and first recognized that white light is actually a mixture of different colors representing the entire visible light spectrum. White light originates from a variety of natural and artificial incandescent sources, including the sun, chemical reactions (such as fire), and incandescent tungsten filaments. The broad emission spectrum from sources of this type is referred to as thermal radiation. Other sources of visible light, such as gas discharge tubes, are capable of emitting light in narrow, well-defined frequency ranges (representing a single color) that depend upon specific energy level transitions in the source material atoms. Strong perception of individual colors also results from specific absorption, reflection, or transmission characteristics of materials and objects that are illuminated with white light. The visible-ultraviolet light absorption spectrum of a common synthetic dye, Iris Blue B, is illustrated in Figure 6. Solutions of this brilliantly colored organic molecule absorb light in both the visible and ultraviolet regions of the spectrum, and appear to most humans as a rich, medium blue color.
Infrared Radiation - Often abbreviated IR, the large band of infrared wavelengths extends from the far-red portion of the visible light spectrum (around 700-780 nanometers) to about one millimeter in wavelength. With photon energies ranging from approximately l.2 millielectron-volts to slightly less than 1.7 electron-volts, infrared waves have corresponding frequencies between 300 gigahertz (GHz) and approximately 400 terahertz (THz). This type of radiation is associated with the thermal region where visible light is not necessarily detectable or even present. For example, the human body does not emit visible light, but it does emit weak infrared radiation, which is felt and can be recorded as heat. The emission spectrum begins at about 3000 nanometers and ranges beyond the far infrared, peaking at approximately 10000 nanometers.
Molecules of all objects that exist above the temperature of absolute zero (-273 degrees Celsius) emit infrared rays, and the amount of emission generally increases with temperature. Approximately half of the sun's electromagnetic energy is emitted in the infrared region, and household items such as heaters and lamps also produce large quantities. Incandescent tungsten-filament lamps are rather inefficient producers of light, actually emitting more infrared than visible waves.

Common tools that rely on detection of infrared radiation are night vision scopes, electronic detectors, sensors in satellites and airplanes, and astronomical instrumentation. So-called heat-seeking missiles used by the military are guided by infrared detectors. In outer space, infrared wavelengths of radiation map the celestial dust between stars, as evidenced by the large dark patches visible from Earth when viewing the Milky Way Galaxy. In the household, infrared radiation plays a familiar role in heating and drying clothes, as well as allowing the remote control operation of garage doors and home entertainment components.
Infrared photography takes advantage of the near-infrared spectrum to record images on specialized film useful in forensics, remote sensing (such as aerial crop and forest surveys), painting restorations, satellite imaging, and military surveillance applications. Curiously, infrared photographs of sunglasses and other optical surfaces coated with ultraviolet and visible light-blocking agents appear transparent, and reveal the eyes behind seemingly opaque lenses. Infrared photographic film will not record thermal radiation (heat) distribution because it is not sufficiently sensitive to long-wavelength radiation (far-infrared). Presented in Figure 7 are several infrared sensor-generated satellite images of two American cities and Mount Vesuvius, in Italy.
Microwaves - Currently the basis of a widespread technology utilized in millions of households for heating food, microwave spectral wavelengths range from approximately one millimeter to thirty centimeters (or about one foot). The appeal of utilizing microwaves in food preparation results from the fortuitous circumstance that water molecules present in most foods have a rotational resonance frequency within the microwave range. At the frequency of 2.45 gigahertz (12.2 centimeter wavelength), water molecules efficiently absorb microwave energy and subsequently dissipate radiation as heat (infrared). If containers composed of materials that do not contain water are utilized to hold food in the microwave oven, they will tend to remain cool, adding a significant additional convenience to microwave cooking.
Tuning a Radio Wave Receiver
Discover how a variable capacitor is coupled to a simple antenna transformer circuit to tune specific wavelengths in a broadband radiofrequency spectrum.
Start Tutorial »Microwaves constitute the highest frequency radio waves, and are emitted by the Earth, buildings, cars, airplanes, and other large objects. In addition, low-level microwave radiation permeates space, where it is speculated to have been released by the Big Bang during creation of the universe. Higher frequency microwaves are the basis for RADAR, an acronym that stands for RAdio Detecting And Ranging, a transmission and reception technique used in tracking large objects and calculating their speed and distance. Astronomers utilize extraterrestrial microwave radiation to study the Milky Way and other nearby galaxies. A significant amount of astronomical information has been derived from studying a specific emission wavelength (21-centimeters or 1420-megahertz) of uncharged hydrogen atoms, which are widely distributed throughout space.
Microwaves are also employed for transporting information from Earth to orbiting satellites in vast communications networks, for relaying information from ground-based stations over long distances, and in terrain mapping. Surprisingly, some of the first electromagnetic experiments conducted by Heinrich Hertz, Jagadis Chandra Bose, and Guglielmo Marconi (the father of modern radio), were carried out using radiation in or near the microwave region. Early military applications capitalized on the narrow beamwidth and increased modulation bandwidth allowed by focusable microwaves, which were hard to intercept and contained relatively large amounts of information. There is some controversy in the scientific community over the potential health risks of cancer and thermal tissue damage associated with continual and cumulative microwave radiation exposure from cellular telephone towers, leaking microwave ovens, and the act of placing mobile telephones close to the brain during use.

Radio Waves - The expansive radiofrequency portion of the electromagnetic spectrum includes wavelengths from about 30 centimeters to thousands of kilometers. Radiation in this range contains very little energy, and the upper frequency limit (about 1 gigahertz) occurs at the end of the band where radio and television broadcasting is confined. At such low frequencies, the photon (granular) character of the radiation is not apparent, and the waves appear to transfer energy in a smooth, continuous fashion. There is no theoretical upper limit to the wavelength of radiofrequency radiation. The low-frequency (60 hertz) alternating current carried by power lines, as an example, has a wavelength of about five million meters (or approximately 3,000 miles). Radio waves used for communication are modulated in one of two transmission specifications: amplitude modulated (AM) waves that vary in the amplitude of the wavelengths, and frequency modulated (FM; see Figure 8) waves that vary in wavelength frequency. Radio waves play important roles in industry, communications, medicine, and magnetic resonance imaging (MRI).
The sound and video portion of television is transported through the atmosphere by shorter radio waves having wavelengths less than a meter, which are modulated for broadcast much like FM radio. Radio waves are also produced by stars in distant galaxies, and can be detected by astronomers using specialized radiotelescopes. Long waves, several million miles in length, have been detected radiating toward the Earth from deep in space. Because the signals are so weak, radiotelescopes are often banded together in parallel arrays containing large numbers of enormous antenna-based receivers.
The nature of the relationship between the frequency (the number of oscillations per unit time) and wavelength (the length of each oscillation) of light becomes apparent when studying the broad range of the electromagnetic radiation spectrum. Very high-frequency electromagnetic radiation, such as gamma rays, x-rays, and ultraviolet light, comprises very short wavelengths and a significant amount of energy. On the other hand, lower frequency radiation, including visible, infrared, microwave, and radio waves, has correspondingly longer wavelengths with lower energies. Although the electromagnetic spectrum is commonly described as traversing about 24 orders of magnitude in frequency and wavelength, there are no intrinsic upper or lower boundaries to the wavelengths and frequencies of this continuous distribution of radiation.



