数字图像数据与生物标本之间的关系
本白皮书探讨了生物标本信号与显微镜数码相机数据之间的联系。了解这种关系可以帮助您设置最佳的图像采集条件,从而获得最高质量的图像和更准确的数据。
数字影像基础
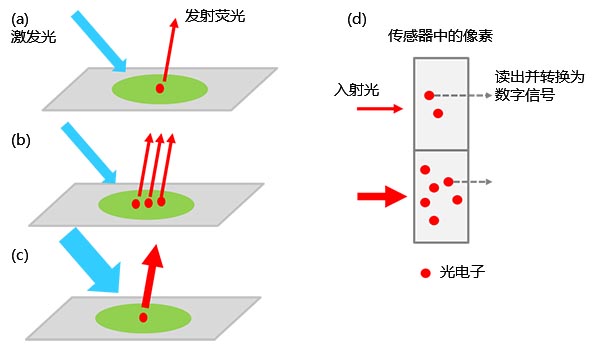
显微镜单色相机是一种可以检测并可视化生物标本光信号的设备。显微镜观察到荧光染料或荧光蛋白所发射的荧光信号,一般通过相机进行收集,相机再将光信号转换为光电子作为数字信号进行检测。
检测到的信号值受多个参量综合影响,包括经过标记的靶分子的数量、激发光强度、荧光激发效率和检测效率(包括相机从光到数字信号的转换效率)等(图1)。
当您对不同的标本使用相同的设备和相同图像采集设置时,上述提到的参数中除了靶分子数量以外,其它参数均为恒定值,换言之,检测到的信号值与靶分子数量成正比。这就意味着,您可以对基因编辑标本和野生型对照组标本进行定量比较。
图1 – 从标本到数字信号的转化:(a)被激发的荧光标记分子发射荧光。 |
信号与背景噪声剖析
本小节将着重介绍影响图像质量的核心因素,以及精心拍摄图像的重要性。
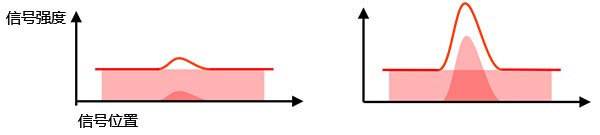
有效信号与背景信号:相机所检测到的信号包含有效信号和背景信号(背景噪声)。要从背景信号中识别出检测目标,您需要让有效信号强度与背景信号强度的比值足够高(图2),这就是信噪比(Signal-to-noise ratio, SNR)。获得更高的信噪比可以带来更高质量的图像和更准确的定量分析。
通常情况下,最大限度增加有效信号(例如,使用更高数值孔径的物镜)和最大限度降低背景信号(例如,使用暗室,更低制冷温度或更高量子效率的相机)是提高信噪比的常用方法。
需要注意的是,改变相机的增益设置并不会提高信噪比,因为它会同时改变有效信号和背景信号。
图2 – 左:低信噪比:背景噪声导致实际弱信号难以被识别。 |
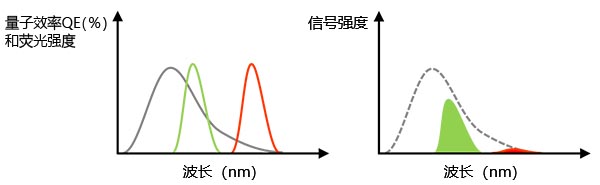
有效信号:如前所述,改善信噪比的一个方法是使用较高数值孔径的物镜,而另一个方法是使用高量子效率(QE)的相机。量子效率代表相机把采集到的光子转换为光电子的效率。切记,如果相机在特定波长下的量子效率为零,则相机将无法感知该波长的光强。例如,当我们为了防止串色而使用近红外染料如Cy7时,必须选择具有在720nm或更长波段的近红外区间感光能力的相机。
图3 – 左:灰线代表相机在检测各波长光线的量子效率。绿线和红线代表荧光染料的发射光谱。右图:检测到的总信号等于左图量子效率和荧光光谱的乘积(即坐标图中的绿色、红色面积大小)。在这个例子中,虽然红色荧光信号足够强,但对应波长范围的量子效率低,因此实际检测到信号值也非常弱。 |
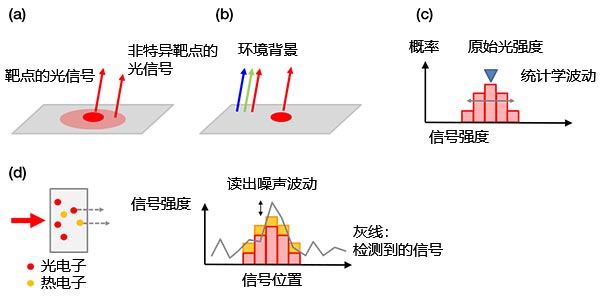
背景噪声:背景信号可分为:
a)生物背景信号
b)非生物背景信号
c)光电子的统计学波动(散粒噪声)
d)相机噪声
散粒噪声非常独特,与抛硬币类似。正如尽管显示“正面”的概率为50%,但仍可能会在两次掷硬币时都得到“ 背面”一样,任何次数为N试验的统计波动均为±√(N)。所检测到的光电子数遵循相同的原理。
所有背景噪声示例如下面的图4中显示。
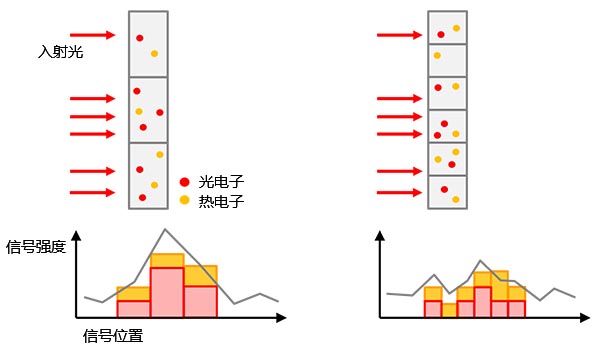
图4 – 背景噪声示例:(a)来自非特异性染色或自发荧光的生物发光背景,(b)载玻片上反射的室内环境光,(c)散粒噪声,(d)包含传感器热电子的相机噪声(左)和读出噪声(右)。可以通过传感器制冷的方式减少热电子。 |
分辨率:使用较大的像素尺寸或像素合并的方式采图可以捕获更多光信号从而提高信噪比,但是增大像素尺寸会降低图像分辨率(图5)。考虑与光学分辨率匹配的最佳像素尺寸。
图5 – 左:使用较大的像素尺寸可以获得更高的感光灵敏度,但会降低图像分辨率。 |
使用显微镜相机的最佳做法
尽管理想的图像采集设置会根据不同应用和标本而变化,但激发光强度和曝光时间这两个常见参数的平衡至关重要。更长的曝光时间或更强的激发光可获得更明亮的荧光,进而获得更高的信噪比,但这些操作也会增加光毒性。这就提出了一个重要问题:如何设置最佳的图像采集参数,可以降低激发光造成的细胞损伤,从而完成更长时间的活细胞成像实验呢?
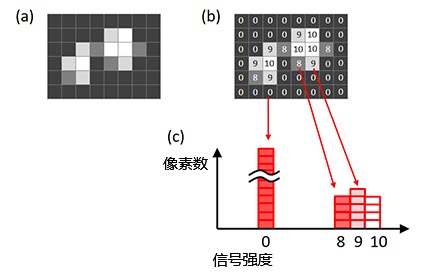
若要确定理想的曝光时间,我们可以使用图像直方图。直方图的X轴为信号强度,Y轴代表了与图像中每个X轴数值对应的像素数量(图6)。
图6 – 图像的直方图(a)原始图像,(b)原始图像中每个像素的信号强度, |
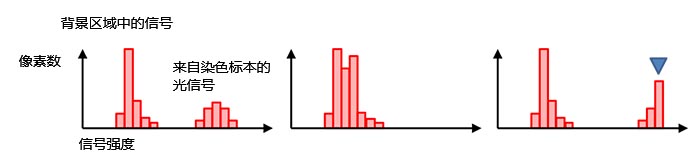
一般情况下,即使不存在背景光,图像黑色背景区域的的像素信号值也不等于零(图7,左)。这样可以避免因图4(d)所述的读出噪声波动导致产生负值信号。直方图的形状和分布可以告诉我们当前的曝光时间是否合适。如果直方图在低信号范围内过于拥挤,则曝光时间太短(图7,中)。如果在最大信号处出现陡峭的“断崖”,则信号值已达到饱和(图7,右)。这种情况下,可以降低激发光强度或缩短曝光时间。
图7 – 正常曝光(左)、曝光不足(中)、 |
一些图像采集分析软件具有自动调节显示(display adjustment)功能,可以在保持原始图像数据的同时获得更佳的展示效果。在大多数情况下,单色相机的信号动态范围(如16位= 65,536阶)要远大于显示器的动态范围(通常为8位= 256阶)。
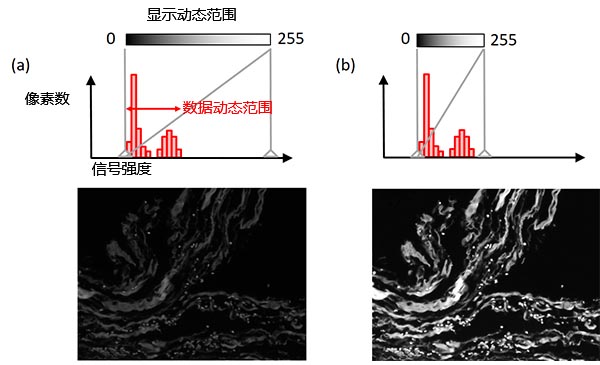
调节显示功能可以改变信号强度和显示亮度之间的关联。通常标本中最强的信号也会远低于相机可处理的最大强度。对此,让显示动态范围与数据动态范围(从背景水平到最亮信号的范围)相匹配,就可以在不改变原始图像数据的同时让图像展示效果更佳(图8)。而使用直方图可以更方便地进行这样的显示调整。
图8 – 显示调整:(上)直方图中灰色垂直线代表了当前显示的动态范围,(中)显示亮度与信号强度之间的关系,(下)示例图像。左例图:原始显示设置。右例像:在保持原始图像数据不变的同时调节显示设置,图像展示效果更佳。 |
设置显微镜相机采集参数的六大步骤
接下来我们总结一下正确设置显微镜相机的六大步骤。需要注意的是,最佳操作步骤取决于您的具体应用和实际标本。
- 确定观察倍率。
- 调整标本的对焦,找到感兴趣的区域。进行这一步操作时,为缩短操作时间并最大限度降低光毒性,可以考虑在相机上使用更高的增益或像素合并模式。另外,我们还建议通过使用自动或手动调节显示在最佳状态下观察信号,并在不观察图像时关闭激发光光闸。
- 找到感兴趣区域后,重新设置增益和像素合并模式。
- 尝试使用最低的激发光强度,并检查是否可以在当前曝光时间内观察到信号。如果无法识别信号,或信噪比过低,可以尝试延长曝光时间。
- 如果曝光时间过长或者超出相机所允许的最大曝光时间,可以逐步尝试更高的激发光强度。
- 检查直方图确认信号没有过饱和。
结论
尽管显微成像的效果受多种因素影响,但了解数字成像的基础知识和技巧可以帮助您为每次实验确定最佳的采集参数。不论针对什么应用和实验,最大限度增强信号、降低背景噪声以及优化采集条件是提高实验数据质量的基本策略。
作者
 |
Takeo Ogama
Scientific Solutions Division OLYMPUS CORPORATION OF THE AMERICAS |
对不起,此内容在您的国家不适用。