Multimode imaging and fluorescence multiplexing have become essential methods to efficiently examine different elements of samples in one experiment. But the quantitative results from this experiment rely on one thing—your ability to extract rich image data.
The Importance of Whole Slide Imaging for Quantification
The basic principle of whole slide imaging is to digitally assemble high-magnification images with a small field of view into a larger overview of the sample. Why do this?
Quite simply, the power of whole slide imaging, sometimes called digital slide imaging or virtual microscopy, is to give a more complete biological context to a microscopic image. Seeing one or two cells in isolation at high magnification can be informative—but placing that cell in the larger context of whole tissues offers broader avenues of investigation.
Then why not just take a macro image of the tissue? The power of whole slide imaging is its macro-to-micro view, where the microscopic resolution enables greater quantification of images, whether it’s counting nuclei throughout a tissue or tracing axons over large distances in a brain.
Because of this powerful combination of resolution and biological context, automated whole slide imaging has become a cornerstone of many research programs. Keep reading to learn about its powerful observation capabilities for extracting new information from your samples.
Imaging Capabilities on Automated Whole Slide Scanning Systems
For years, whole slide imaging systems were limited in their imaging modes. Today, automated whole slide imaging systems like our VS200 research slide scanner can combine multiple observation methods, enabling you to view structures only visible under certain conditions. This means you can mix and match observation methods like:
- Brightfield: in brightfield imaging, transmitted white light passes through the sample, and tissues are visualized through chromogenic stains. But brightfield will not provide any innate contrast unless you are using chromogenic stains like Hematoxylin and Eosin.
- Epifluorescence: enables specific antibody labeling and higher degrees of molecular targeting. It also permits a higher number of stains to be overlaid, which expands the field of multiplexing.
- Phase contrast and polarization: phase optics and polarization can be included in whole slide images, providing various levels of innate contrast based on phase ring illumination or polarized light.
- Darkfield: uses the refractive index differences in membranous structures in tissue. Although some stains can enhance darkfield, the technique can be completely label-free and provide a level of contrast comparable to fluorescence. However, this observation mode can be sensitive to dust.
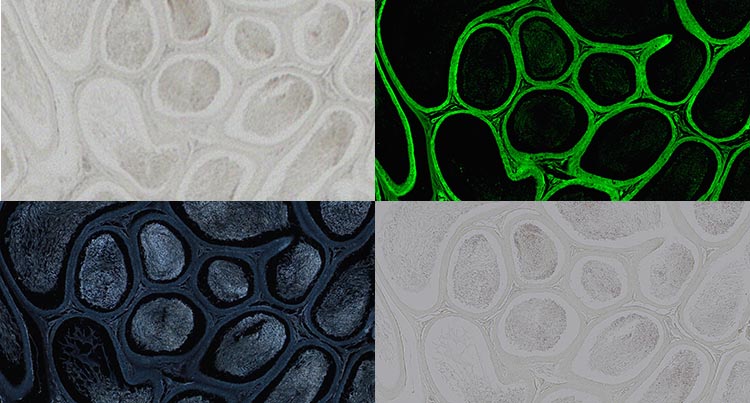
Testis in Paramount, non-stained, captured with a 20X objective. Brightfield (top left), fluorescence (top right), darkfield (bottom left), polarization (bottom right). Image data courtesy of Robin Wacker, Günthersleben, Germany.
But that’s not all—the latest virtual slide scanners come with other imaging tools to help you extract better data from your experiment. For instance, our VS200 slide scanner can focus through multiple planes of tissue to:
- Acquire multiple planes at the highest resolution
- Image samples up to 100 µm thick
- Capture images using all available observation methods
- Easily obtain information from all dimensions of your sample
- Calculate projections (deconvolution, maximum Z, or EFI)
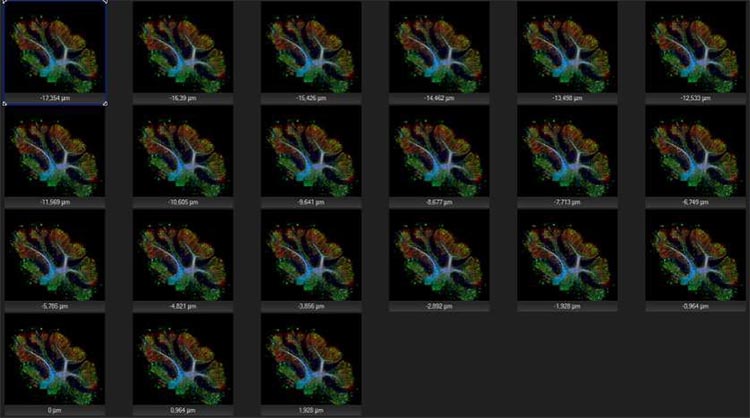
Z-stacking can acquire up to 35 planes at the highest resolution, making it easy to obtain information from all dimensions of your sample.
Another helpful tool is the automatic oil dispensing mechanism, which helps the system achieve the highest resolution.
Combining these different imaging modes and features is even more powerful when used with the molecular specificity afforded by antibody labeling techniques. Yet, it can still be difficult to efficiently and quantitatively label with fluorescence.
With that in mind, our next section will cover imaging technologies that can help with fluorescence multiplexing applications.
Technology Essentials for Tissue Multiplexing
Fluorescence multiplexing has become a useful tool in immuno-oncology research due to the incredibly complex tumor microenvironment, but it requires specialized software, personnel, and equipment to get an assay up and running and applied to a tumor of interest.
Manufacturers have developed new technologies that address the different needs and issues that kept this type of assay from being widely used. These technological must-haves include:
High-level multiplexing that addresses:
| Workflow compatibility that offers:
|
Whole slide imaging that captures:
| Software that can identify complex phenotypes:
|
An Overview of the UltiMapper™ Multiplex Marker Assay
Another helpful technology for multiplexed imaging experiments is the UltiMapper I/O assay.
UltiMapper I/O assays use InSituPlex® technology to enable whole-slide, high-resolution multiplexing for cell phenotyping and spatial profiling of biomarker activity. As a preoptimized, reagent-based solution, these kits seamlessly integrate into existing equipment and software across the immunohistochemistry (IHC) workflow and enable researchers to go from staining to image acquisition and assessment in just one day.
The image below shows the simple 4-step process:
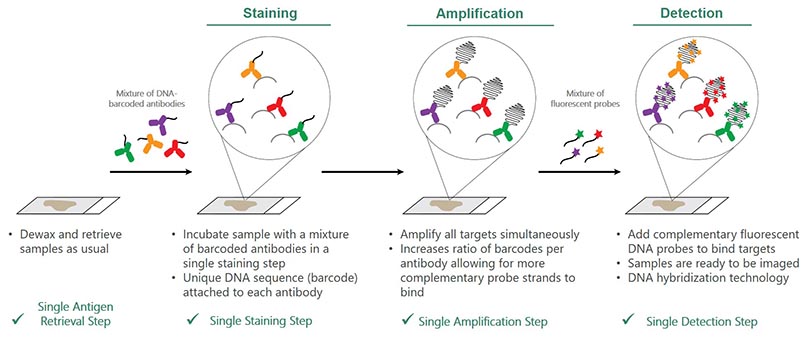
Step 1: Retrieve:
- Dewax and retrieve samples as usual
Step 2: Stain
- Incubate sample with a mixture of barcoded antibodies in a single staining step
- Unique DNA sequence (barcode) attached to each antibody
Step 3: Amplify
- Amplify all targets simultaneously
- Increases ratio of barcodes per antibody, allowing for more complementary probe strands to bind
Step 4: Detect
- Add complementary fluorescent DNA probes to bind targets
- Samples are ready to be imaged
- DNA hybridization technology
3 Factors That Influence Image Quality for Quantification
When designing your experiment, keep in mind that the right combination of optical and staining technologies will lead to the best downstream analysis. Try as you might to analyze low-quality images from a bad reagent set or poor acquisition and optical configuration, you’re just not going to achieve a good analysis.
Here are three optical factors that influence image quality for quantification:
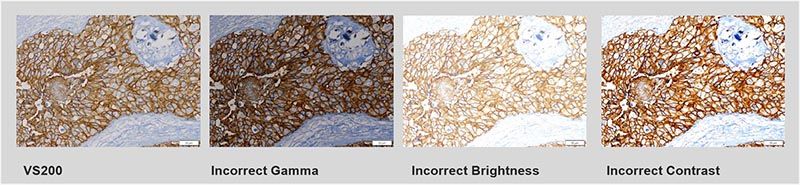
- Accurate illumination color: It’s important to have a reproducible and true color representation in H&E staining. Transmitted optics should have spectral characteristics that mimic halogen light sources to reproduce purple, cyan, and pink colors.
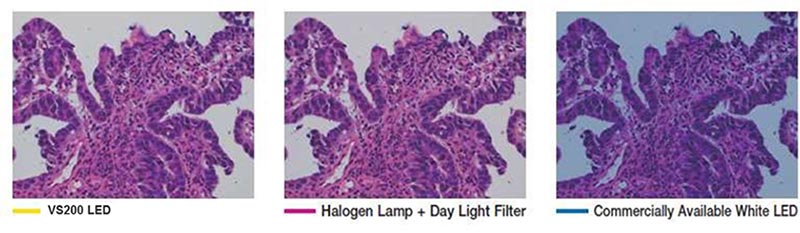
The VS200 system’s true color LED for transmitted illumination has the same spectral characteristics and power as a halogen lamp, so purple, cyan, and pink stains are correctly represented, imaged, and rendered.
- Color fidelity and precision: In addition to having the right illumination spectral characteristics, the microscope camera should have excellent color fidelity and precision. Make sure to use a color-corrected camera with the right balance to accurately represent the tissue. Eyeballing digital images can be subjective as different acquisition settings lead to different image outcomes, but color-corrected cameras and ICC profiles replicate excellent color and intensity on computer monitors.
- Flatness of field: Capturing a series of images where the field is not flat, or there was no correction built in, can cause a halo effect through every field of view, which is a problem for quantification. In this case, you must use non-linear balancing and filtering of every field of view, which takes time and resources. Consider the optical configuration that will help maximize the flatness of field to lead to a better quantitative result.
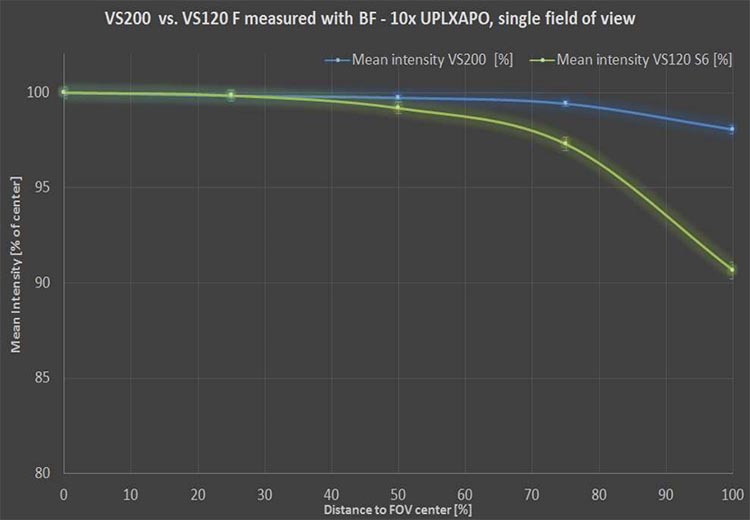
High-performance X Line™ objectives on the VS200 researcher scanner help improve image flatness.
Final Thoughts
Because images can be acquired in volume with a multiple focus Z-stack, you can improve and enhance the images’ contrast using methods like deconvolution, maximum intensity projections, or extended focus views, which enable you to select the best focus for the image.
Ultimately, the solution to quantitative analysis is a combination of good labeling, good optics, and software that is robust enough for segmentation analysis, garnering statistical information that is biologically relevant.
To learn more about extracting rich data from your multimode imaging and fluorescence multiplexing experiments, tune into my on-demand webinar here.
Related Content
5 Ways the VS200 Slide Scanner Can Benefit Your Research
.jpg?rev=E220)