Matching Filter Blocks with Probes
Matching Filter Blocks with Probes
The essential feature of any fluorescence microscope is to provide a mechanism for excitation of the specimen with selectively filtered illumination followed by isolation of the much weaker fluorescence emission using a second filter to enable image formation on a dark background with maximum sensitivity. These conditions are satisfied in modern fluorescence instruments by a combination of filters that coordinate excitation and emission requirements based on the action and properties of the dichromatic beamsplitter.
The anatomy of a typical fluorescence filter block is diagrammed schematically in Figure 1, along with the associated spectral profiles of the dichromatic mirror, excitation, and barrier filters. Filter blocks are usually assembled with custom tools supplied by the manufacturer so the operator can interchange filters and the dichromatic mirror. The excitation and barrier filters are secured into place with retainer clips, optical glue, or circular threaded mounts (see Figure 1). In general, these filters can be removed without opening the optical block because they are positioned over recessed apertures on the flat exterior surfaces. Replacing a dichromatic mirror is more difficult and requires complete disassembly of the block to gain access to the interior. Most block sections are cast with a 45-degree diagonal joint that serves double duty by protecting the interior and supporting the dichromatic mirror at the proper angle. After removing the fasteners holding the block sections together (pins or small screws), the mirror can be removed by loosening or moving the retainer clip, and then very carefully dropping it out of the block. The dichromatic mirror should be handled with caution because the interference coating is usually not protected and can be easily scratched. Several of the filter manufacturers who supply the microscope companies also offer a wide selection of aftermarket filters and dichromatic mirrors for a variety of fluorescence applications.
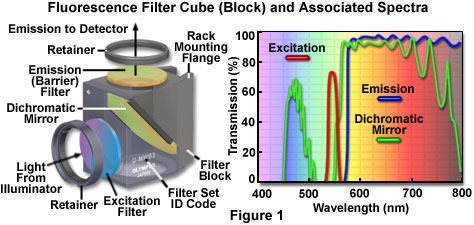
Fluorescence filter designs include longpass, shortpass (edge filters), and the narrow, medium, and wide family of bandpass filters. Examples of several common filter profiles are illustrated by the spectra presented in Figure 1, and a catalog of current Olympus filter combinations is listed in Table 1. The emission filter spectrum (blue curve) in Figure 1 is produced by a longpass interference filter having a cut-on wavelength of approximately 575 nanometers. Longer wavelengths are transmitted through the filter, while shorter wavelengths are blocked. A narrow bandpass excitation filter from the same set (red curve, Figure 1) has a bandwidth of approximately 20 nanometers, while the dichromatic mirror (green curve) has transmission regions approximating a medium (455-490 nanometers) and wide bandpass filter (560-775 nanometers). Because the dichromatic mirror effectively serves as a longpass filter in the green, yellow and red regions of the visible spectrum (560 to 700 nanometers), it is treated as such in filter sets. A working knowledge of how fluorophore absorption and emission spectral profiles can be utilized to select the appropriate filter set for fluorescence microscopy is essential to successful implementation of this technique.
Dichromatic mirrors (or beamsplitters) are the most critical component in a fluorescence microscopy filter combination, and resemble longpass interference-type filters that are fabricated to close tolerances with multiple layers of dielectric materials. The major difference between a dichromatic mirror and a standard interference filter is that the mirror is specifically designed for reflection and transmission at defined boundary wavelengths, and must operate at a 45-degree angle with respect to the microscope and illuminator optical axes. Dichromatic mirrors are positioned with the interference coating facing the excitation light source in order to reflect short excitation wavelengths at a 90-degree angle through the optical train to the specimen. The same mirror must also act as a transmission filter to pass long wavelength fluorescence emission from the objective to the image plane. Because the wavelength transition region between almost total reflection and maximum transmission is often limited to only 20 or 30 nanometers, the dichromatic mirror is able to precisely discriminate between excitation and emission wavelengths.
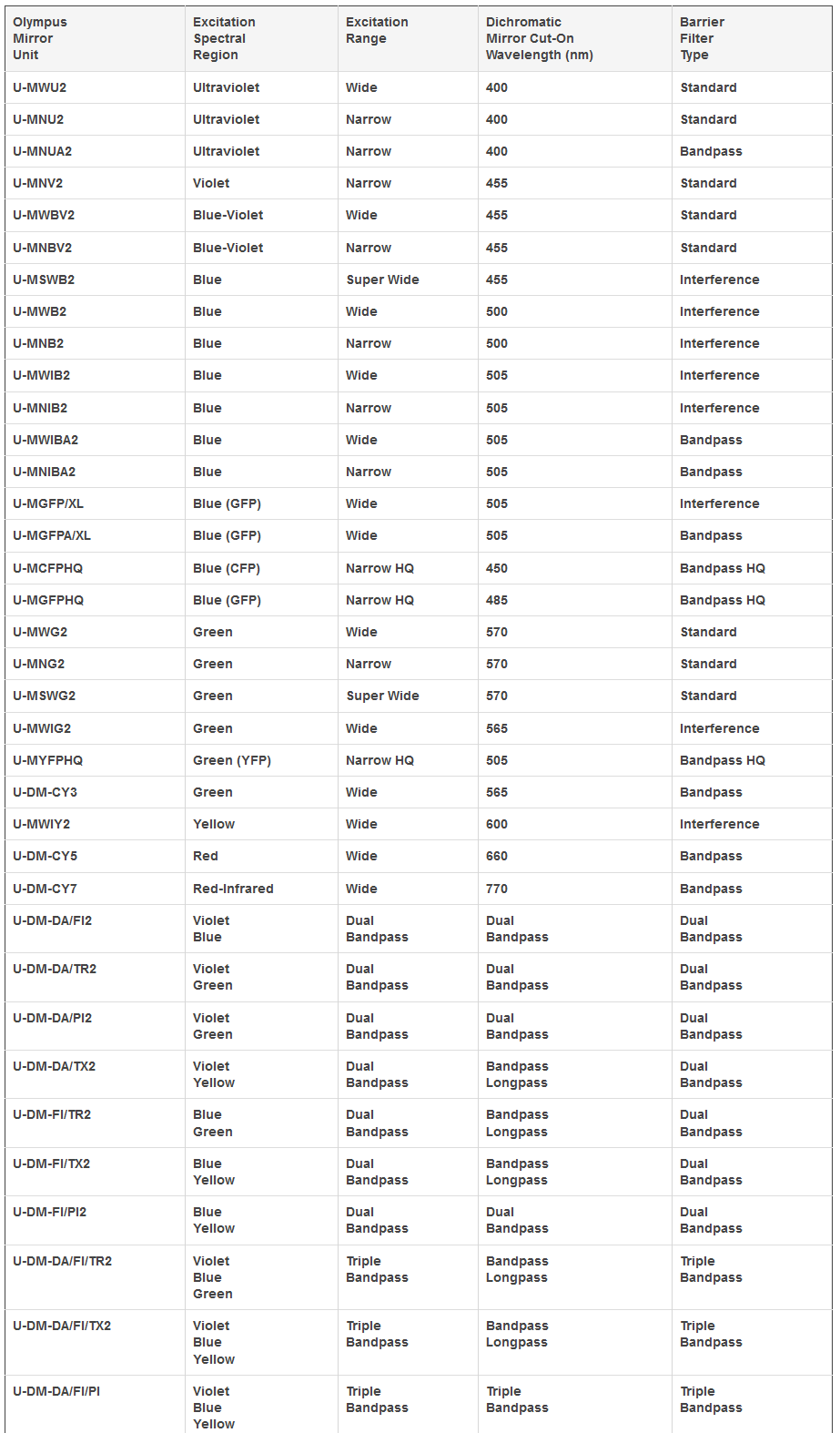
Olympus Universal Fluorescence Mirror Unit Specifications
Fluorescence filter sets are designed so that a particular band of excitation wavelengths exactly matches the reflection region in the dichromatic mirror. The result is efficient reflection of excitation light through the microscope and onto the specimen. Fluorescence emission from the specimen must match a high transmission region in the dichromatic mirror in order to enable these wavelengths to pass through to the detector. The barrier filter is less important in the overall scheme, but still plays an important role in ensuring that scattered and reflected excitation wavelengths, fluorescence from probes other than the target, and general background intensity due to autofluorescence is removed. The most important factor in creating a filter set is to make certain that the transmission, reflection, and emission profiles of the involved filters match in the appropriate regions. Otherwise, excitation wavelengths might be able to pass through the dichromatic mirror and fog the image, or fluorescence emission can inadvertently reflect at the mirror to compromise image brightness.
Even in seemingly perfectly matched filter combinations, slight overlaps between spectral profiles of the individual filters can occur to diminish performance. Of particular concern is crosstalk between the excitation filter and the dichromatic mirror, which enables some of the excitation light to pass through the mirror and reflect from the walls of the filter block. Light reflected at highly oblique angles can be partially transmitted through the barrier filter, as discussed above, to reduce image contrast. This type of leakage through filters is termed bleed-through or crossover, and occurs to a greater or lesser extent with practically all filter combinations. One of the primary areas of concern with both filter manufacturers and microscope companies is to improve the design of fluorescence filter combinations in order to reduce the level of crossover.
Sorry, this page is not
available in your country.



