Large assemblies of macromolecules through which the cell can anchor itself to the extracellular matrix are known as focal adhesions. In general, the cell connects with the surrounding substrate through the protein integrin, which links to actin filaments, the cytoskeletal components that give the shell its structural integrity. The connection of focal adhesions to the outside world makes them ideally suited for transmitting mechanical forces and signals containing information about the surrounding conditions. In the digital video presented above, rabbit kidney epithelial cells (RK-13 line) are expressing a fusion of tandem dimer Eos (tdEos) fluorescent protein to human vinculin.
Video 1 - Run Time: 06 Seconds
Video 2 - Run Time: 07 Seconds
Video 3 - Run Time: 11 Seconds
Video 4 - Run Time: 09 Seconds
Video 5 - Run Time: 11 Seconds
The ERM proteins are named for the principle elements: ezrin, radixin, and moesin. These proteins cause the cytoplasm’s focal adhesions to bind to actin filaments for anchorage to the surrounding medium. One end of these proteins must bind to an integral membrane protein, while the other binds to actin structures within the cell. When the anchoring proteins are activated, both of these distinct domains become exposed and ready for bonding. However, in the inactive state, the molecule folds up, which renders both domains inactive. The catalyst for change in the activation status is most likely a phosphorylation process of the ERM proteins. In the digital video presented above, rabbit kidney epithelial cells (RK-13 line) are expressing a fusion of tandem dimer Eos (tdEos) fluorescent protein to human vinculin.
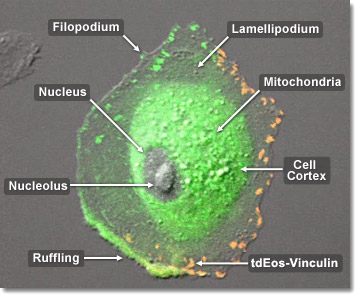
A cell is free to change shape as well as divide and differentiate as soon as it is attached to the substrate through focal adhesions. At this point the cell typically ceases to produce additional adhesions, while focal adhesions allow cells to send and receive signals to and from the extracellular matrix. The cell is anchored internally to the surrounding matrix when transmembrane proteins, called integrins, bind to extracellular molecules, such as fibronectin, linking them with an inner cluster of proteins connected to actin filaments. The behavior of the cell is influenced by chemical and mechanical signals, which traverse this interconnected protein network. The integrin group matures into a full focal adhesion through a series of mechanical tensions between the outer matrix and the transmembrane-integrin molecular complex, in conjunction with multiple phosphorylation reactions. In the digital video presented above, rabbit kidney epithelial cells (RK-13 line) are expressing a fusion of tandem dimer Eos (tdEos) fluorescent protein to human vinculin.



