During my time with Olympus, I’ve been amazed by our customers’ creative confocal microscope setups—from DIY-made sample holders to experimental apparatuses. And since our FV3000 confocal microscope comes in both upright and inverted configurations, we can accommodate and orient samples in many ways.
The FV3000 microscope is designed for fluorescence observations. However, with a little ingenuity, it can also perform reflectance confocal imaging for some cases. The reflectance confocal imaging technique is often overlooked despite its versatility and practicality, so I’ll discuss a smart way to use it to help you get the most out of your system.
I’ll highlight how reflectance confocal imaging can be used to extract endogenous information from biological tissues.
Reflectance Confocal Imaging of Fibrillar Biomolecular Complexes
One use for a reflectance light path is capturing endogenous signals for added contrast and awareness of the sample environment.
Here’s how it works: the interface between biological fibrillar protein structures and water produces a strong reflective contrast signal. By simply de-scanning the excitation light to a TruSpectral detector that is set to an overlapping bandwidth, you can identify specific cellular structures, the organization state of the extracellular matrix environment, or material surface features.
Conveniently, the FV3000 confocal microscope features a completely spectral-based light path, so every channel can be used for reflectance detection. When combined with your fluorescence observations, this added multispectral reflectance capability can extract the location of unlabeled cellular structures.
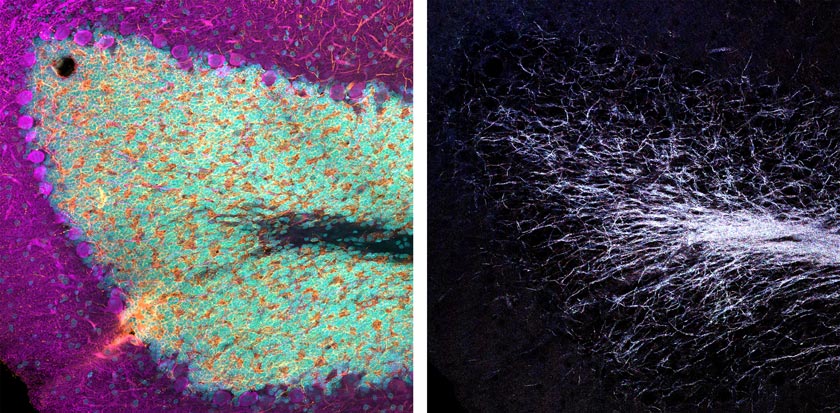
Consider this example: I’ve imaged an immunofluorescence-labeled fixed rat brain section slide provided by EnCor Biotechnology, Inc. As you can see in the images below (Figure 1), a standard three-channel VBF acquisition reveals excellent epitope-specific labeling of neuronal dendrites (orange), catecholaminergic neurons (magenta) that contain dopamine or norepinephrine, and dye intercalation that provides our nuclei stain (cyan).
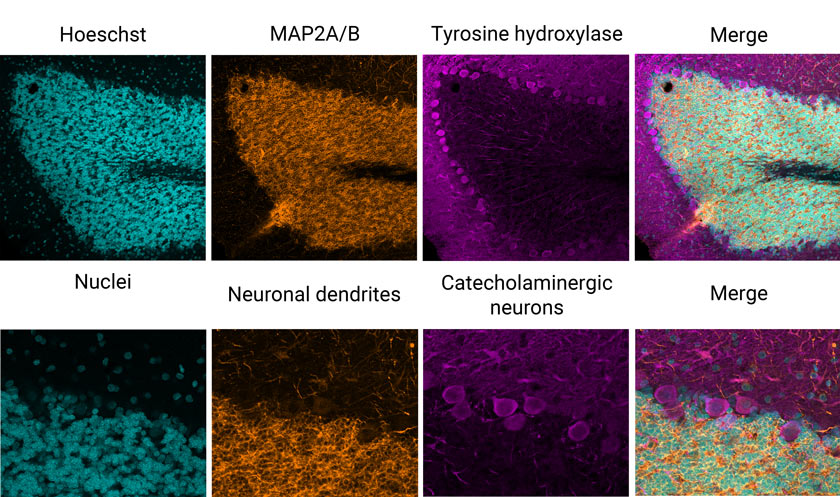
Figure 1: Three-color immunofluorescence-labeled rat brain section stained with antibodies from EnCor Biotechnology, Inc. Top panels: whole field single Z-plane image acquired with an X Line 20X objective. Bottom panels: true pixel resolution of a highlighted inset square.
Despite the strong and broad-spectrum fluorescence background created by the immunofluorescence preparation, we can use our reflectance skills to tweeze out more endogenous information.
By using four channels simultaneously to image the reflectance of laser lines 405, 488, 561 and 640 nm, we can create a multispectral map of the myelinated neurons within this tissue. You can see what this looks like in the images below (Figures 2 and 3).
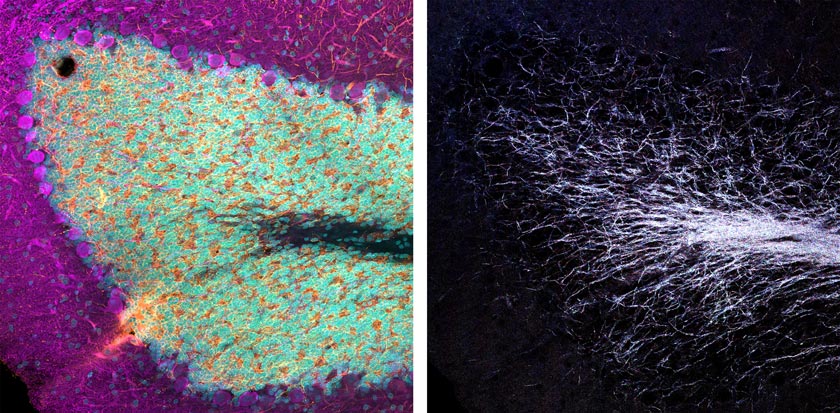 Figure 2 (left): Three-channel merge of the immunofluorescence-derived signal shown in Figure 1. Right: the same field of view four-channel merged image of simultaneously acquired reflectance confocal imaging using laser lines 405, 488, 561, and 640 nm. | 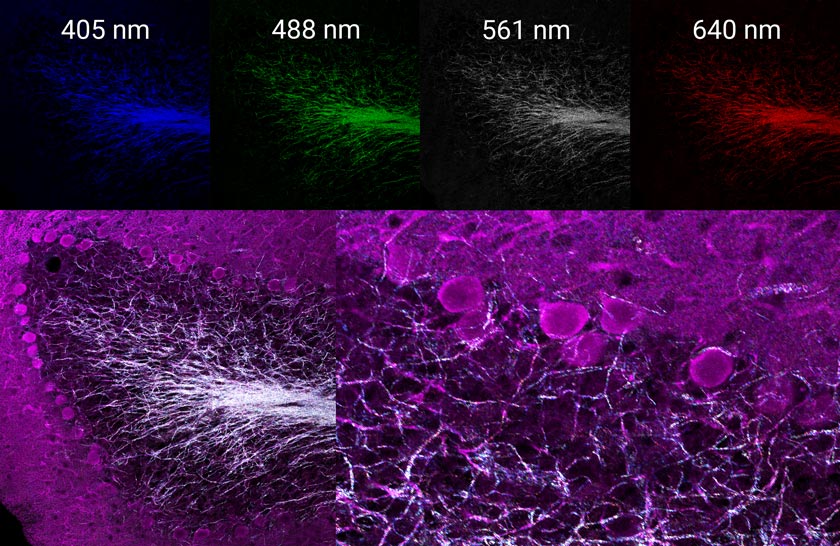 Figure 3 (top): Individual reflectance confocal images of each line acquired simultaneously. Bottom: merged images of immunofluorescence-labeled dopamine-containing neurons and the multispectral reflectance confocal image of endogenous axon myelination. |
You can use this label-free information to judge the degree of myelination occurring on the neurons of interest that were selectively labeled by immunofluorescence or to help with applications that require axon tracing.
Reflectance confocal imaging can also be used to elucidate the extracellular matrix of 3D cultured tissue models. As you can see in the images below (Figure 4), neovessels growing during angiogenesis interact closely with the surrounding collagen fibrils of the stromal environment.
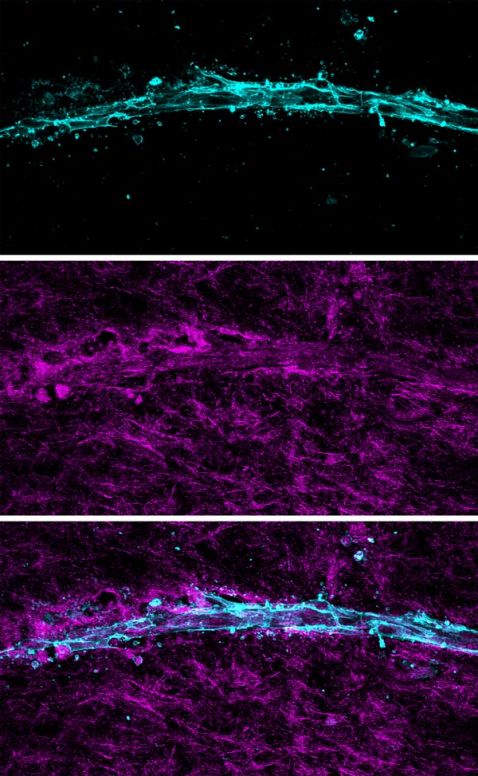
Figure 4: Z-projection of a 3D-printed blood vessel within a collagen matrix. Top: the fluorescence confocal image of rhodamine-labeled endothelial cells. Middle: 405 nm reflectance confocal image showing the fibrillar collagen network and areas of remodeling proximal to the vessel. Bottom: the merged image. Scale bar equals 100 µm.
By using Advanced Solutions Life Sciences’ Angiomics™ isolated microvessels to recapitulate native angiogenesis in vitro with the FV3000 confocal microscope, it’s clear that angiogenic neovessels (visualized via confocal fluorescence) reorganize the immediately adjacent collagen fibrils (visualized using reflectance imaging) as they grow through the 3D stromal environment.