Entwicklung einer neuen Fucci(CA)-Anwendung: Fluoreszenzsonde zur Visualisierung von Zellzyklen
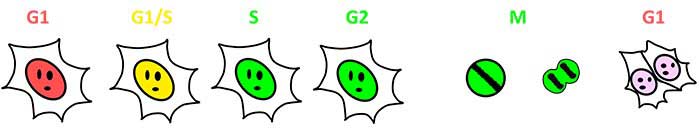
Fucci (ein fluoreszierender, auf Ubiquitin basierender Zellzyklusindikator) ist ein genetisch codiertes Set aus zwei Fluoreszenzsensoren zur Beobachtung des Zellzyklus lebender Zellen. Fucci (CA), das 2017 neu entwickelt wurde, markiert Zellkerne je nach Zellzyklus entweder mit mCherry (rot) oder mVenus (blau). Fucci (CA) weist während der gesamten G1-Phase eine deutliche Expression von mCherry und eine abrupte Abnahme der roten Fluoreszenz am Ende der G1-Phase auf. Mit Fucci (CA) lässt sich eine kurze G1-Phase zuverlässig erkennen und S- und G2-Phasen sind differenzierbar, was früher schwierig war.
Fucci (SA) |
|
Fucci (CA) |
|
Abb. 1: Visualisierung des Zellzyklusverlaufs durch Fucci (SA) und Fucci (CA)
Geringe Fototoxizität, Zeitraffer-Bildaufnahme von undifferenzierten ES-Zellen
Undifferenzierte embryonale Stammzellen (ES) vermehren sich rasch und sind sehr empfindlich. Die Fototoxizität während der Zeitrafferbildaufnahmen kann ES-Zellen schädigen und ihre Proliferationsgeschwindigkeit verringern, so dass es schwierig ist, Zeitrafferbilder von ES-Zellen unter physiologisch genauen Bedingungen durchzuführen. Das Mikroskop FV3000 ermöglicht aufgrund des hocheffizienten Lichtweges und der empfindlichen Detektionsgeräte den Einsatz einer extrem niedrigen Laserstärke mit geringer Fototoxizität für Zeitrafferaufnahmen. Dank dieser Eigenschaften des FV3000 konnte eine Forschungsgruppe einen Versuch mit Zeitrafferaufnahmen über 57 Stunden durchführen und so drei normale Zellzyklen sich schnell teilender, undifferenzierter ES-Zellen vollständig erfassen.
Related Videos |
Abb. 2: Zeitrafferbetrachtung von mit Fucci (CA) 2.1 markierten Maus-ES-Zellen
Bildgebungsbedingungen
Probe: Murine ES-Zellen
Objektiv: Silikon-Immersionsobjektiv (UPLSAPO30XS)
Mikroskop: FLUOVIEW FV3000
Laser: 445 nm (AmCyan), 594 nm (mCherry)
Erkennung einer kurzen G1-Phase
Fucci (CA) markiert die gesamte G1-Phase mit einem starken roten Signal, so dass eine präzise Messung der Phasengrenzen auch bei schnell proliferierenden, undifferenzierten ES-Zellen möglich ist. Durch Zeitrafferaufnahmen auf Einzelzellniveau wurde festgestellt, dass murine ES-Zellen (mESCs) mit einer Verdoppelungszeit von etwa 11 Stunden und einer G1-Phase von nur 1 Stunde proliferieren.
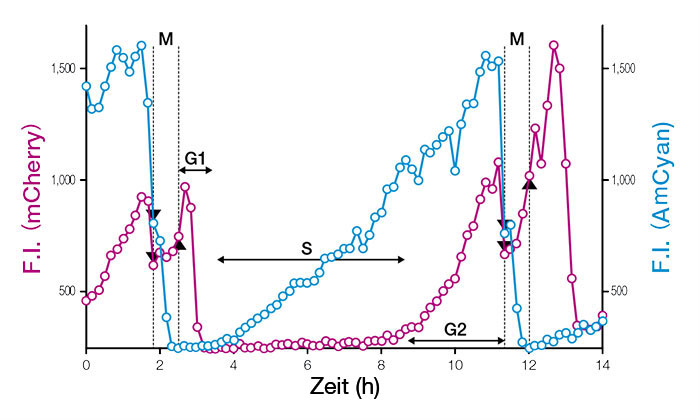
Abb. 3: Zeitprofile der Fluoreszenzintensitäten (F.I.) von Einzelzellkernen mit Expression von Fucci (CA) 2.1
Für diesen Versuch genutzte Funktionen des FV3000
Vollspektrum-System mit hoher Empfindlichkeit
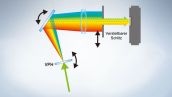
In der Serie FV3000 kommt die TruSpectral-Detektionstechnologie von Olympus zur Anwendung, bei der das Licht durch Transmission durch eine Volumen-Phasen-Hologramm-Einheit gebeugt wird. Diese Technologie ermöglicht einen wesentlich höheren Lichtdurchsatz als herkömmliche spektrale Detektionseinheiten mit Reflexionsgittern.
Hohes Signal-Rausch-Verhältnis (S/N) bei schwachem Anregungslicht

Die hochempfindliche Detektoreinheit des FV3000 verfügt über bis zu vier Kanäle mit GaAsP-Photovervielfacherröhren (PMTs), die schwache Fluoreszenzsignale mit einer maximalen Quanteneffizienz von 45 % erfassen. Zusätzlich reduziert die Peltier-Kühlung in diesen Detektoren das Hintergrundrauschen um 20 %. Diese Merkmale ermöglichen in ihrer Kombination eine Bildgebung mit hohem Signal-Rausch-Verhältnis, selbst bei schwachem Anregungslicht.
Aufnahme physiologisch genauer Bilder des Zellzyklus: Kommentar von Dr. Asako Sakaue-Sawano
Undifferenzierte mESCs teilen und bewegen sich in einem dreidimensionalen (3D) Zellkulturraum, so dass zur Beobachtung ihrer Proliferation eine XYZT-Bildgebung erforderlich ist. Bei Zeitrafferbildaufnahmen kann eine wiederholte Laserabtastung in undifferenzierten mESCs aufgrund von deren hoher Anfälligkeit für Fototoxizität möglicherweise Zellzyklusveränderungen induzieren. Mit dem Mikroskopsystem FV3000 konnten Dr. Asako Sakaue-Sawano und ihre Kollegen schonende 4-dimensionale (XYZT) Bildgebungsversuche durchführen und den gesamten Zellzyklus schnell proliferierender murinen ES-Zellen mit sehr kurzer G1-Phase auf Einzelzellebene genau charakterisieren.
Danksagungen
Dieser Anwendungshinweis wurde durch Mitwirkung folgender Forscher erstellt:
Laboratory for Cell Function Dynamics, RIKEN Center for Brain Science
Dr. Masahiro Yo |
Dr. Asako Sakaue-Sawano |
Dr. Atsushi Miyawaki |
Referenz
Weitere Einzelheiten zu den in diesem Anwendungshinweis erwähnten Studien finden Sie in folgendem Artikel:
A. Sakaue-Sawano, et al. „Genetically Encoded Tools for Optical Dissection of the Mammalian Cell Cycle.” Molecular Cell, volume 68, issue 3 (October 2017): pp. 626–640.e5.
Verwendete Produkte
wurde erfolgreich zu Ihren Lesezeichen hinzugefügt
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.