Desenvolvimento de uma nova aplicação de Fucci (CA): uma sonda fluorescente para visualização de ciclos celulares
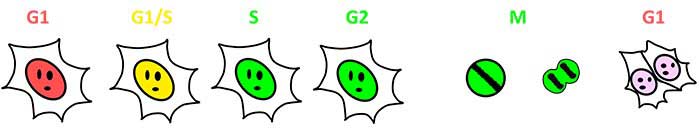
O Fucci (indicador de ciclo celular fluorescente baseado em ubiquitinação) é um conjunto geneticamente codificado de dois sensores fluorescentes para monitoramento de ciclos celulares vivos. O Fucci (CA), recentemente desenvolvido em 2017, rotula núcleos com mCherry (vermelho) ou mVenus (azul) de forma dependente do ciclo celular. O Fucci (CA) produz uma expressão abundante de mCherry ao longo da fase G1, com um decréscimo abrupto de fluorescência vermelha no fim da fase G1. O Fucci (CA) pode ser usado para detectar, de forma confiável, uma fase G1 curta e distinguir a fase S da fase G2, uma distinção que, anteriormente, era difícil de conseguir.
Fucci (SA) |
|
Fucci (CA) |
|
Figura 1: Visualização de uma progressão do ciclo celular por Fucci (SA) e por Fucci (CA)
Aquisição de imagens com lapso de tempo e fototoxicidade baixa de células-tronco embrionárias indiferenciadas
As células-tronco embrionárias indiferenciadas proliferam rapidamente e são muito delicadas. A fototoxicidade durante a aquisição de imagens com lapso de tempo pode danificar as células-tronco embrionárias e reduzir sua velocidade de proliferação, dificultando a execução da aquisição de imagens com lapso de tempo das referidas células em condições fisiológicas exatas. O microscópio FV3000 permite uma aquisição de imagens com lapso de tempo e fototoxicidade baixa usando uma potência do laser extremamente baixa, graças a um caminho da luz altamente eficiente e a dispositivos de detecção sensíveis. Essas propriedades do FV3000 permitiram que um grupo de pesquisa efetuasse um experimento de aquisição de imagens com lapso de tempo, com uma duração de 57 horas, no qual foram abrangidos três ciclos celulares normais de células-tronco embrionárias indiferenciadas de divisão rápida.
Vídeos relacionados |
Figura 2: Observação com lapso de tempo de células-tronco embrionárias de rato rotuladas com Fucci (CA) 2.1
Condições de aquisição de imagens
Amostra: células-tronco embrionárias de rato
Objetiva: objetiva de imersão em silicone (UPLSAPO30XS)
Microscópio: sistema FLUOVIEW FV3000
Laser: 445 nm (AmCyan), 594 nm (mCherry)
Detecção de uma fase G1 curta
O Fucci (CA) rotula completamente a fase G1 com um sinal vermelho forte, o que permite uma medição precisa dos limites da fase, mesmo em células-tronco embrionárias indiferenciadas de proliferação rápida. Usando a observação com lapso de tempo no nível de célula única, constatou-se que as células-tronco embrionárias de rato (mESCs) proliferam com um tempo de duplicação de cerca de 11 horas e uma fase G1 de apenas uma hora.
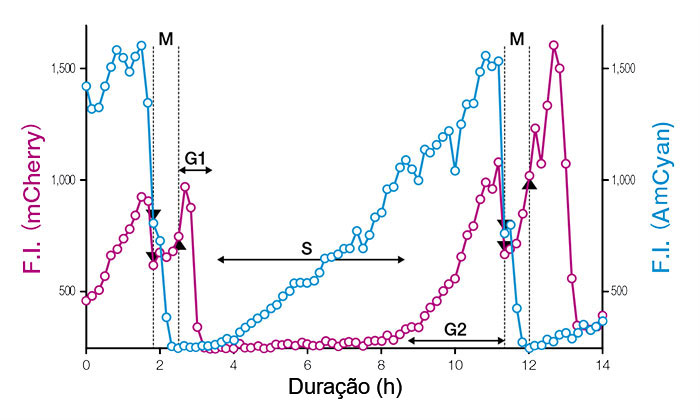
Figura 3: Perfis temporais de intensidades de fluorescência (IF) de núcleos de células únicas expressando Fucci (CA) 2.1
Características do FV3000 neste experimento
O sistema totalmente espectral proporciona alta sensibilidade
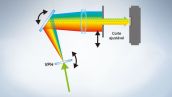
A série FV3000 usa a tecnologia de detecção TruSpectral da Olympus que difrata a luz por transmissão, por meio de uma unidade de holograma de fase volumétrica. Essa tecnologia, quando comparada a unidades de detecção espectral convencionais com grades de tipo refletivo, permite um rendimento de luz muito superior.
Alta relação sinal-ruído sob luz de excitação baixa

A unidade do detector de alta sensibilidade no FV3000 possui até quatro canais de tubos fotomultiplicadores (PMTs) GaAsP que capturam sinais fluorescentes fracos com uma eficiência quântica máxima de 45%. Além disso, o resfriamento Peltier nesses detectores reduz o barulho de fundo em 20%. Combinadas, essas características permitem uma aquisição de imagens de alta relação sinal-ruído, mesmo com uma luz de excitação baixa.
Aquisição de imagens fisiologicamente exatas do ciclo celular: comentário da Dra. Asako Sakaue-Sawano
As mESCs indiferenciadas dividem-se e movem-se em um espaço de cultura celular tridimensional (3D), exigindo uma aquisição de imagens XYZT para monitorar sua proliferação. Durante a aquisição de imagens com lapso de tempo, uma varredura a laser continuada pode induzir alterações no ciclo celular em mESCs indiferenciadas devido à sua alta susceptibilidade a fototoxicidade. O sistema do microscópio FV3000 permitiu que a Dra. Asako Sakaue-Sawano e seus colegas efetuassem experimentos de aquisição de imagens quadridimensionais (XYZT) cuidados para caracterizar com exatidão o ciclo celular completo com uma fase G1 muito curta de células-tronco embrionárias de rato de proliferação rápida no nível de célula única.
Agradecimentos
Esta nota de aplicação foi preparada com a ajuda dos seguintes pesquisadores:
Laboratório de Dinâmicas da Função Celular, Centro RIKEN para Ciências do Cérebro
Dr. Masahiro Yo |
Dr. Asako Sakaue-Sawano |
Dr. Atsushi Miyawaki |
Referência
Para obter mais detalhes sobre os estudos mencionados nesta nota de aplicação, consulte os seguintes artigos:
A. Sakaue-Sawano, et al. “Genetically Encoded Tools for Optical Dissection of the Mammalian Cell Cycle.” Molecular Cell, volume 68, edição 3 (outubro de 2017): pp. 626–640.e5.
Produtos usados nesta aplicação
foi adicionado com sucesso aos seus favoritos
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.