Dr. Stephanie Shiers is a research scientist at the University of Texas at Dallas, where she completed her PhD in cognition and neuroscience in 2019. Her translation studies investigate the preclinical mechanisms and therapies for chronic pain using donated human nervous tissue from the Southwest Transplant Alliance.
Stephanie’s microscopy images have been selected in several research journals, including most recently on the cover of Science Translational Medicine (Feb. 16, 2022). We sat down with Stephanie to uncover what it takes to create cover-worthy works of art.

Dr. Stephanie Shiers’ cover art on Science Translational Medicine (vol. 14, issue 632) (reprinted with permission from AAAS).
Q: Who or what helped inspire you to become a scientist?
Stephanie: I triple majored and minored because I had no clue what I wanted to do. I was set on medical school originally. However, I realized I didn't want to do medicine after taking an emergency medical technician (EMT) training course. I then added neuroscience and history because I thought maybe I'll like humanities, want to be a lawyer, or do neuroscience and be a PhD. I still didn't know once I finished my undergraduate degree what I wanted to do for a career until I joined a lab on campus. And I really liked it!
I eventually applied for an immunohistochemistry job at the UC Davis/NIH NeuroMab facility. The facility generated monoclonal antibodies for use in preclinical research. I loved every part of that job. I loved doing staining, antibody validation, and looking under the microscope. It was rewarding and eye opening. My mentors, Drs. Belvin Gong and Karl Murray, were extremely encouraging and helped me settle on a career path. My time there helped me realize that ‘science is the closest thing we have to magic.’ I tell that to our undergraduate and PhD students now all the time. We are making proteins and mRNAs, and all these things emit fluorescence. We're looking at things you can't see with the naked eye. At one point in time, you will know something that no one else in this world knows. To me, that is magic.
Q: What was your proudest and most memorable microscopy moment?
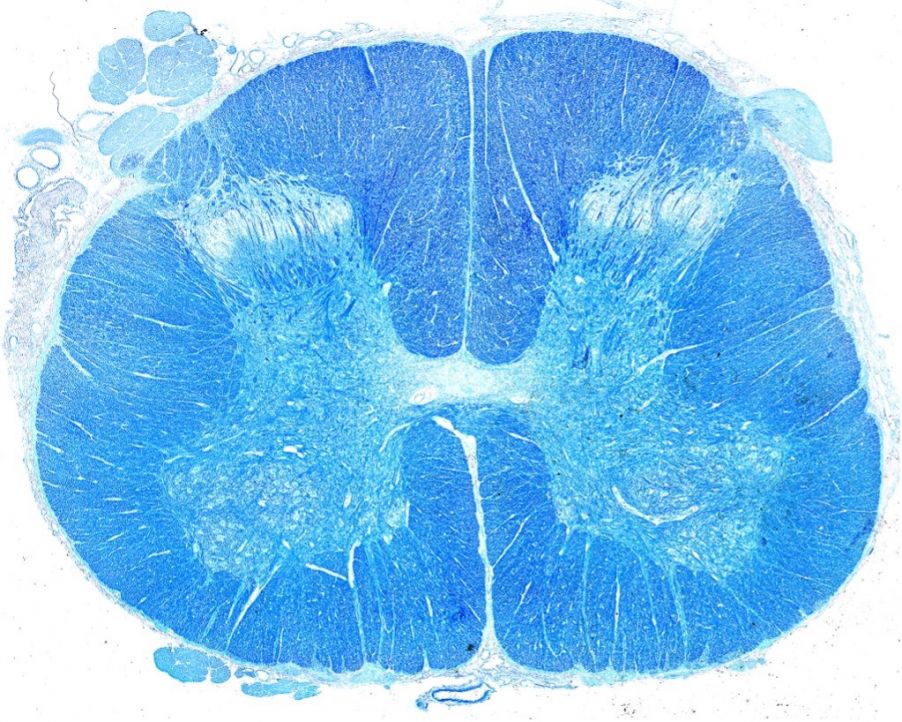
Stephanie: My proudest microscopy moment is when my first paper was published along with my cover image. It was exciting enough to have my first paper published. I wasn’t expecting to also get the cover, as I was just taking pretty pictures for my love of neuroscience art. All my data for the paper was taken with a 40X objective. I decided to take some images with the 100X objective to capture a pretty picture and see it at a higher magnification. We had just opened the Discovery Center with a newly installed FV3000 confocal laser scanning microscope, and I had never tried the system before. I could see all the dendrites and the axon initial segment. It looked striking. When I shared those images with my principal investigator, Ted Price, he encouraged me to apply for the cover art along with submitting my paper.
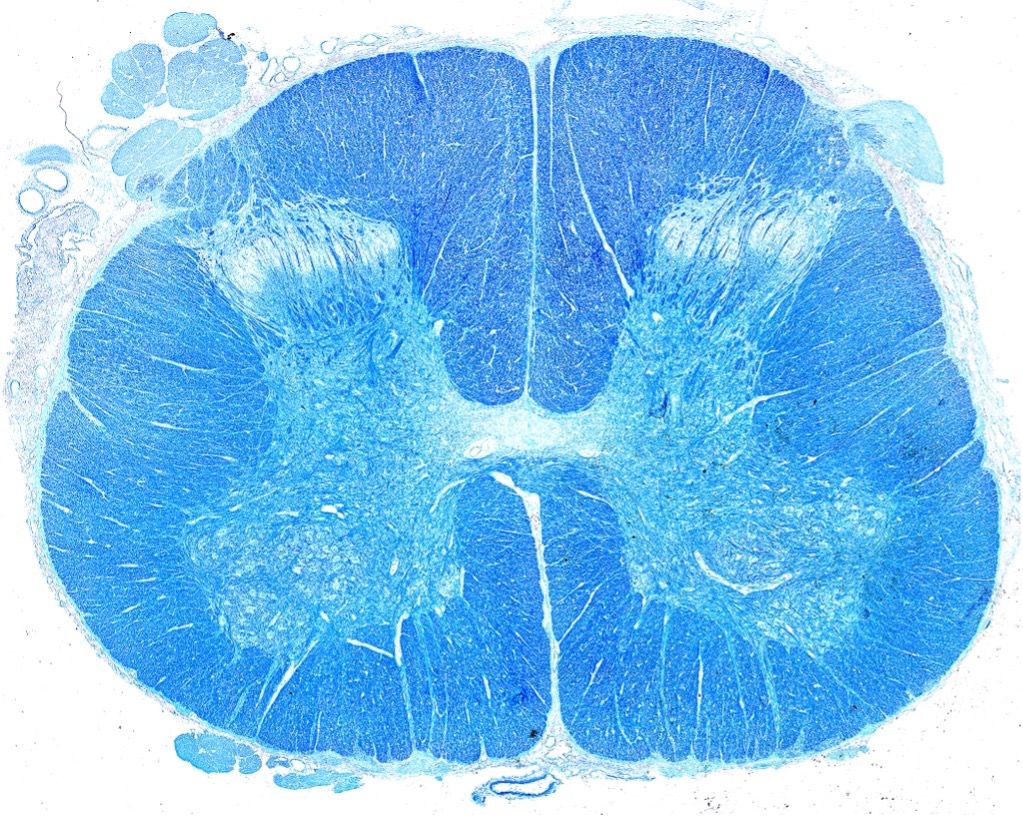
Cover art on research journals captured by Dr. Stephanie Shiers (The Journal of Neuroscience vol. 38, issue 33 (left; reprinted with permission from the Society for Neuroscience) and Science Translational Medicine vol. 13, issue 595 (right; reprinted with permission from AAAS).
My most memorable moment was when I was training a student who had been struggling in the lab to get her staining to work. She had not been trained on microscopy, so we went on the FV3000 confocal microscope together. I will never forget the look on her face. She was blown away. I was elated to see that. It was something I felt during my first imaging experience on the FV1200 system when I started my PhD. It was great to witness someone else experiencing the wonder and amazement of science. That’s what I hope people feel when they see my neuroscience art images for the first time—that spark of magic.
Q: What does it mean to you to have your work on the cover of research journals?
Stephanie: I was elated because it is scary going into a PhD program. You're around all these people who are experts, and I didn’t feel like I knew what I was doing. It was a stressful time but having successful data and a successful representation of your hard work as a journal cover and as a paper was both rewarding and affirming. All that hard work and stress was worth it in the end. It meant the world then. It still does. That first time was amazing, and I still have a video saved from seven years ago that shows me holding up the framed print of my cover art.
Q: What are some of the tips and tricks that would be helpful for someone looking to create similar cover-worthy images?
Pro tip #1: First, having the right instruments goes a long way.
Stephanie: My work at NeuroMab taught me a lot about brightfield imaging and neuroanatomy. When I started my PhD program, I adapted this knowledge to work with immunofluorescence and higher-end imaging systems such as the FV1200/FV3000 confocal microscope and VS200 research slide scanner from Evident. The FV3000 system is the best instrument I have ever worked with! These types of higher-end microscope systems are in a whole other class when it comes to obtaining the best image quality possible and the highest signal to noise compared to what I had used before.
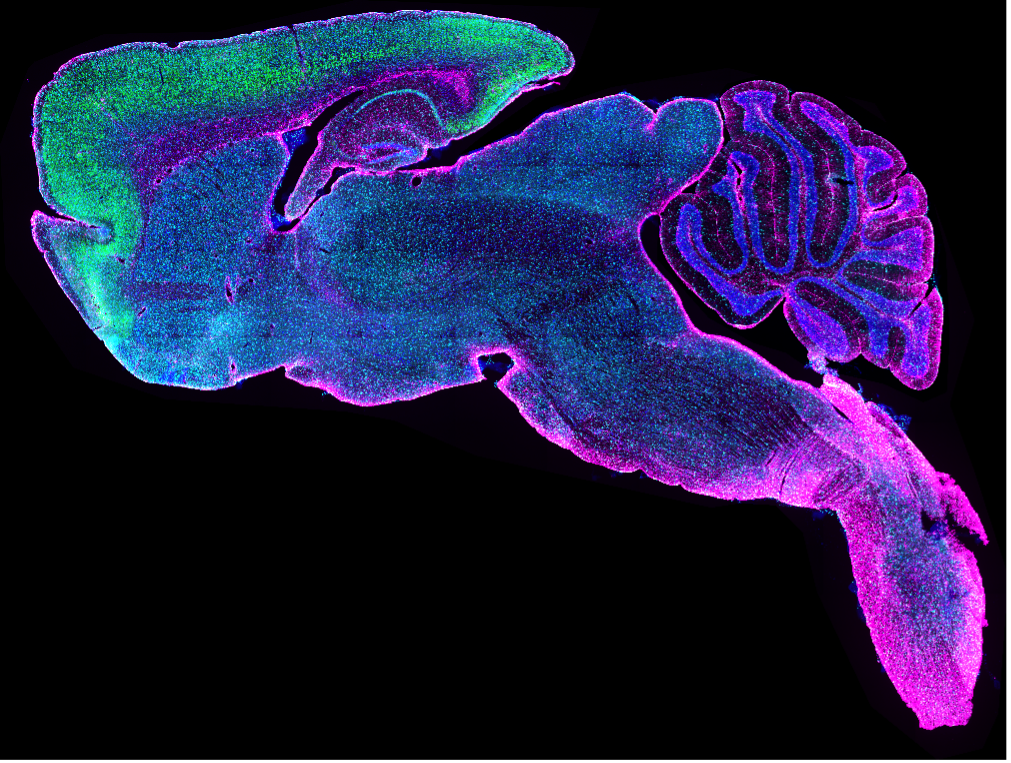
Whole slide image of AMIGO1 (green), IBA1 (cyan), GFAP (magenta), DAPI (blue) staining on a mouse brain sagittal cut. Captured on the VS120 slide scanner by Dr. Stephanie Shiers.
Pro tip #2: Get as much training as possible and build on that knowledge.
Stephanie: Although I had performed a lot of microscopy previously, it was mostly brightfield imaging and immunoperoxidase staining. Because of that, I didn't need a fancy microscope. However, I learned a lot about neuroanatomy, cell specificity, subcellular localization of proteins, and those types of things.
It wasn't until I entered the PhD program that I adapted my previous knowledge to do immunofluorescence staining. I’d say the most important thing for immunofluorescence staining is using proper negative controls because it is so critical for understanding the difference between background and real signal. It’s also extremely important to understand how to use the microscopes properly. The team of experts at Evident trained me on how to use the higher-end systems like the VS120 slide scanner and FV1200/FV3000 confocal microscopes.
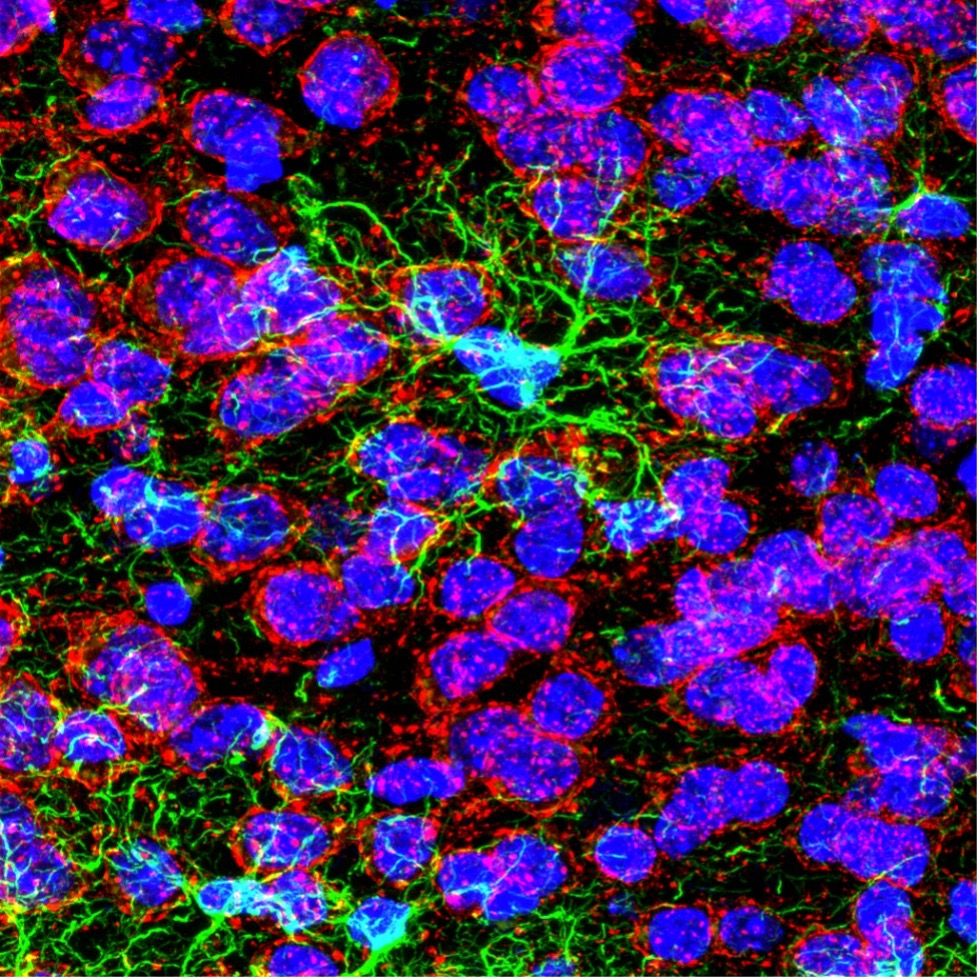
Confocal image of GFAP (green), DAPI (blue), and AMIGO1 (red) staining on a mouse infralimbic cortex. Captured at 100X on the FV3000 confocal microscope by Dr. Stephanie Shiers.
Pro tip #3: Preplan your imaging during data collection.
Stephanie: When I'm imaging for data collection, I always try to preplan my data figures. As a PhD student, I would collect data, analyze for weeks, then realize way down the line after collecting all this other data that I needed to make a figure and take some higher-resolution pictures. Then I would grab the slides to image them, and of course they don’t look the same. You are left wondering: do I have to repeat the experiment? Do I use these lower-quality images in my figures? Now when I image for data collection, I always prepare the figures in my mind, including what images I'm going to use in the research paper. If I find something that's representative of what I'm seeing, I'll take the high-resolution images right then.
For the journal cover, I'll also take high-resolution images, and Z-stacks if necessary. I always take at least one image that looks striking and is high resolution.
Pro tip #4: Experiment and try new things.
Stephanie: There are lots of different stains out there (brightfield pathology stains, tracers, genetic tags, calcium dyes). When these stains are coupled with powerful microscopes, you can easily generate some really abstract, eye-catching images. One of my favorite examples is my work with Eric Bridenbaugh from Evident, who in passing mentioned that the FV3000 confocal microscope was capable of capturing 6-channel immunofluorescence. We both got super excited and, on a whim, decided to just try it out. Six-channel imaging is virtually unheard of due to technical and imaging challenges. Typically, scientists are limited to 3–4 antibodies when doing immunofluorescence staining because of species compatibility issues, and the imaging is limited to 3–4 channels due to the overlap in the emissions profiles of the fluorescence dyes. You essentially can’t parse out each wavelength of light to visualize each stain individually. However, I used a trick I learned at NeuroMab―employing isotype-specific antibodies. With this trick, I could generate a 6-channel stained sample. Eric helped me configure the FV3000 microscope’s TruSpectral™ detectors to capture each stain, and it worked the first time! We generated these beautiful 6-channel stained images, and you can see each structure so clearly. It looks unbelievable―the images are so striking with a rainbow of color!
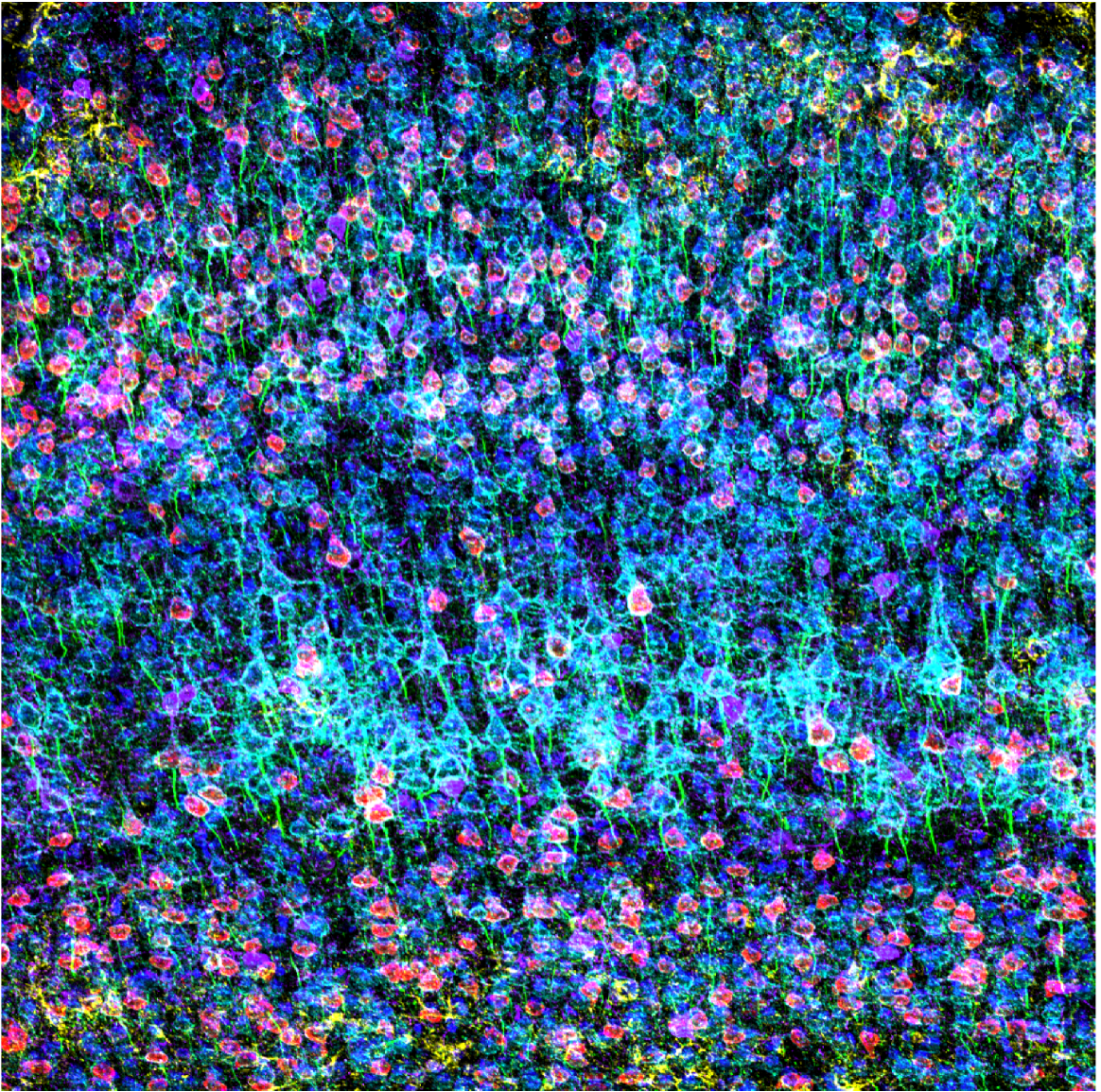
Confocal image of GFAP (yellow), CAMKII (red), AMIGO-1 (cyan), Parvalbumin (purple), AnkG (green), and Nuclear yellow (blue) on a mouse prefrontal cortex. Captured at 100X on the FV3000 confocal microscope by Dr. Stephanie Shiers.
Pro tip #5: Just have fun with it, and don’t forget about stylizing.
Stephanie: I started taking pretty pictures for fun because I love the artistic part of neuroscience images. I took pretty pictures for data collection of course, but sometimes I took pretty pictures just for the sake of taking pretty pictures. That’s how my images came to be. It has resulted in a folder on the lab server with all my best images. I have fun with them. Some images are experiments in Photoshop or changing the colors and brightening and contrasting. There are so many renderings of the same pictures in there. I also like to experiment with new imaging tools and stains. When I started, we had just installed the FV3000 confocal microscope and opened the Discovery Center at UT Dallas. I wanted to start using that microscope and try out the 100X magnification for the first time because we didn’t have it on our other microscopes.
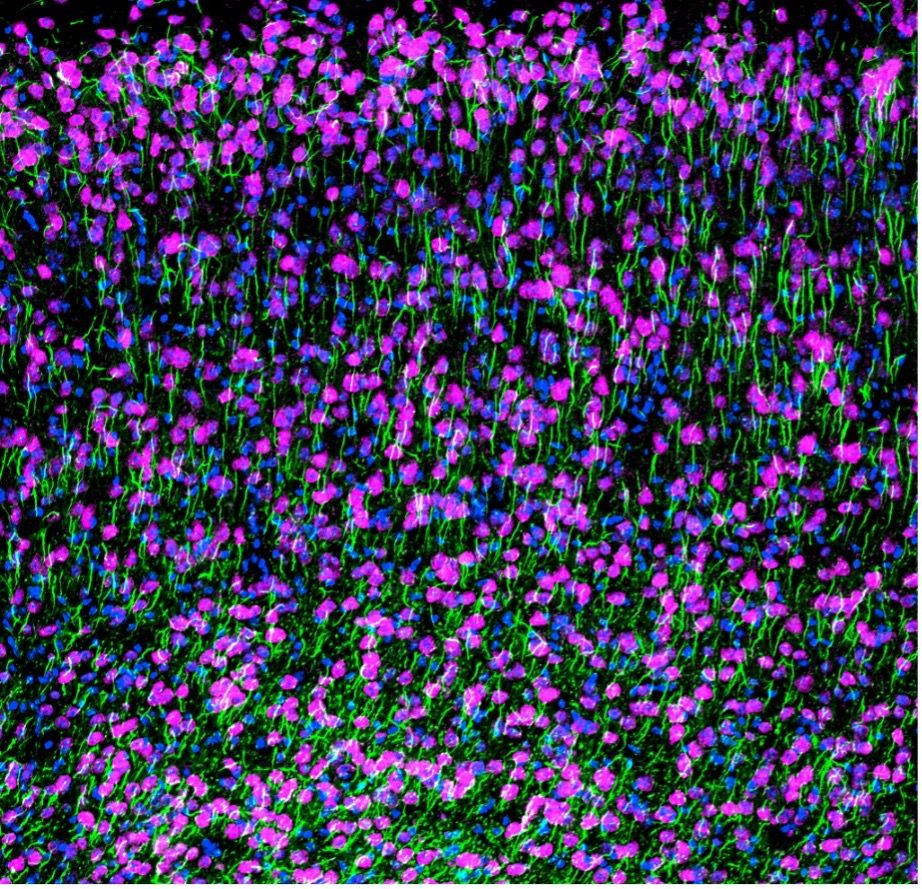
Confocal image of NeuN (magenta), AnkG (green), and DAPI (blue) staining on a mouse cortex. Captured using the FV3000 confocal microscope by Dr. Stephanie Shiers.
Q: What advice would you give to someone who’s trying to get their microscopy images published?
Stephanie: Submit as many images as you can! Many people don't submit cover art as they may think their pictures aren’t worthy of a cover image. Or they may be too excited that their paper was accepted and don’t realize they can also submit a cover image. A lot of journals are eager for cover image submissions.
Also, many people don’t realize that the image for a journal cover does not need to be the exact image they used for data collection. A lot of journal covers can be abstract—almost like drawings. They even have artists make them.
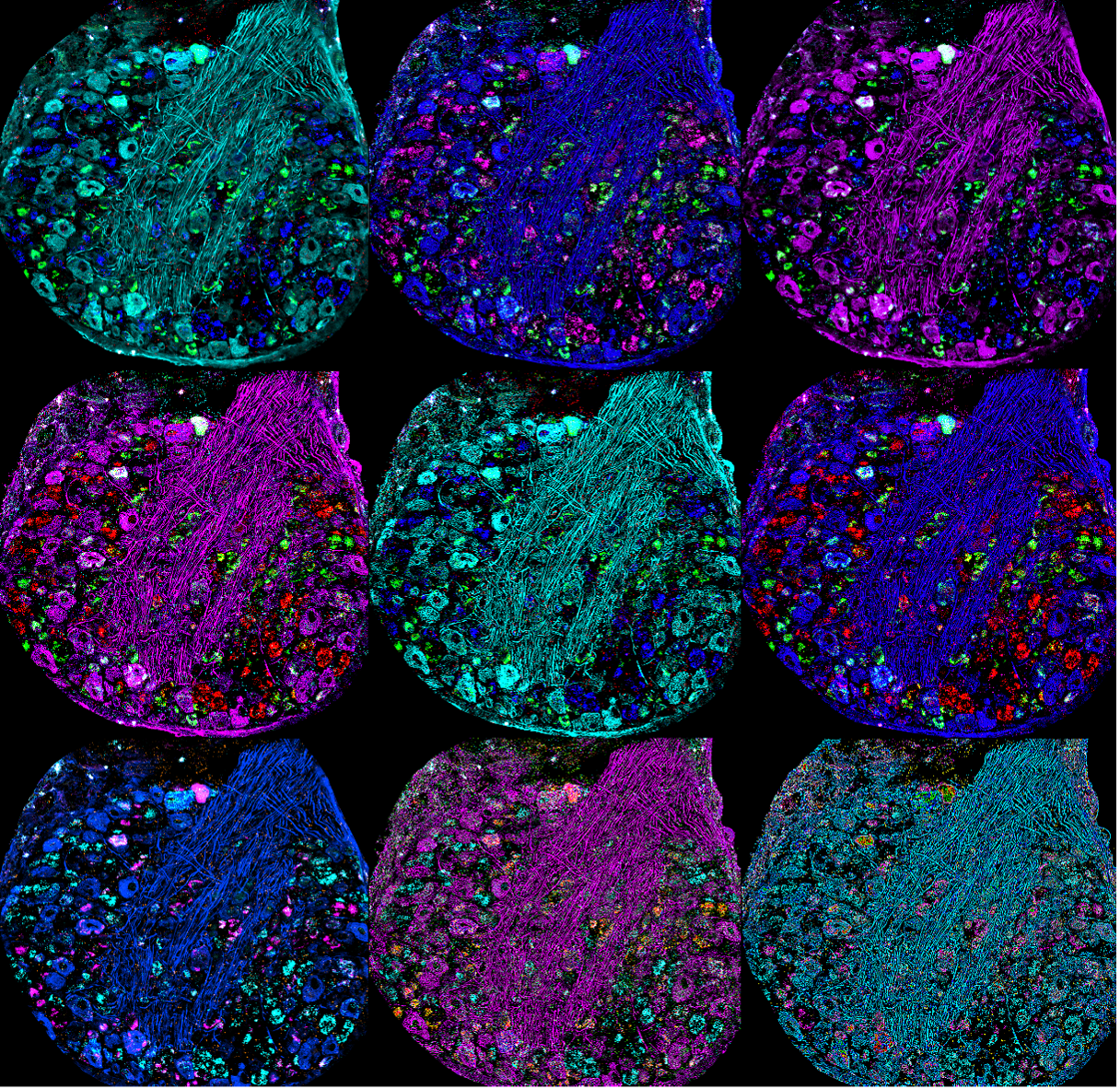
Multiple stylized renderings of a confocal image of a mouse dorsal root ganglion stained for NF200, CGRP mRNA, P2x3r mRNA, and Ifnar2 mRNA. Captured using the FV3000 confocal microscope by Dr. Stephanie Shiers.
Related Content
Multiplexing with the FLUOVIEW FV3000 Confocal Microscope
EVIDENT Workflow Solutions to Make Your Research More Efficient