FV3000共聚焦多色荧光成像
对小鼠内侧前额叶皮层的形态学特征描述
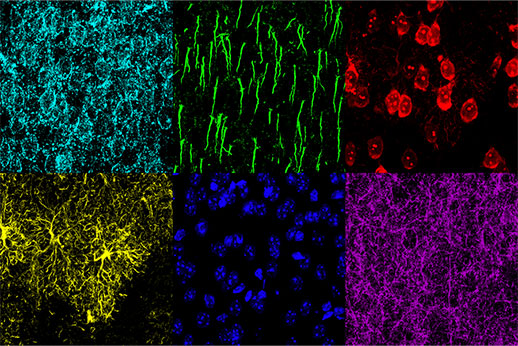
认知功能障碍的机制研究往往需要把大脑形态学变化与生理响应关联起来。在同一个脑片样本上鉴别不同的形态学结构,对于理解病变状态和药物治疗如何影响大脑的形态尤为重要。在本篇研究中,共聚焦显微镜FV3000的全真光谱TruSpectral检测技术的使用成功地在小鼠内侧前额叶皮层(medial prefrontal cortex,mPFC)观察到6种不同的细胞结构,包括小胶质细胞,锥体细胞,抑制型神经元,神经细胞膜,神经细胞轴突起始段和细胞核。
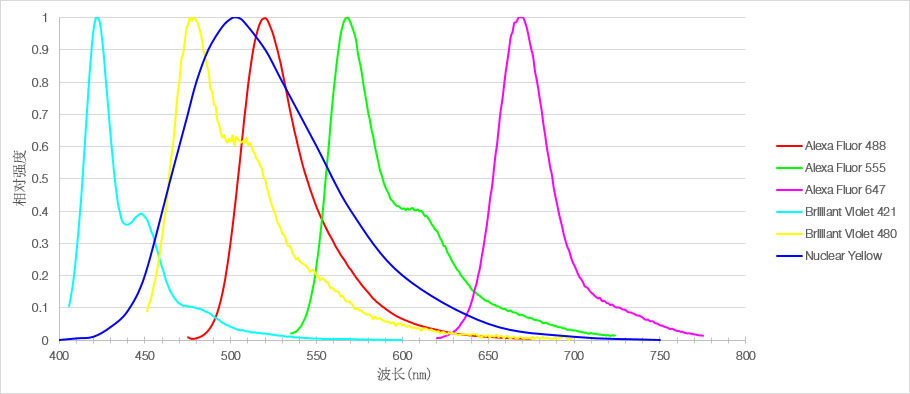
图1 用于标记小鼠mPFC切片的6种荧光染料发光光谱
全真光谱TruSpectral检测技术帮助区分6种不同的形态学结构
在本次实验中,科研人员使用了6种不同的荧光染料分别标记了30μm厚的小鼠内侧前额叶皮层固定标本的不同细胞结构,并使用搭载了UPLSAPO20X物镜的Olympus共聚焦显微镜FV3000进行多色荧光脑切片3D成像。通过全真光谱TruSpectral检测,不仅可以针对不同的荧光染料单独设定最优的采集条件,还能优化检测光谱,避免荧光串色。结果显示每一种荧光染料都有明亮的信号和独特的发射光谱特征,确保了同一种样品的多种结构的检测精度。
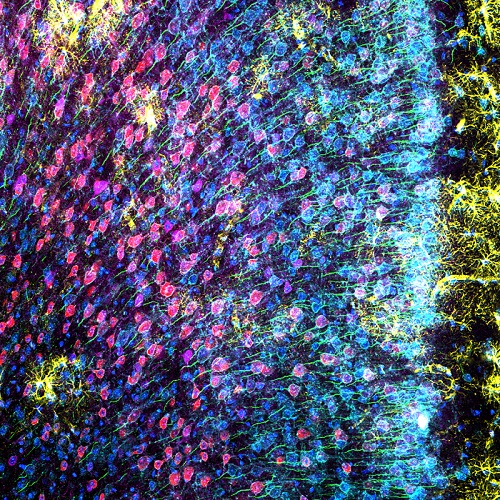
图2 小鼠内侧前额叶皮层切片6色标记:神经胶质纤维酸性蛋白(GFAP,小胶质细胞,黄色),钙调蛋白依赖蛋白激酶Ⅱ(CaMKII,锥体细胞,红色),Amphoterin诱导蛋白1前体(AMIGO-1,神经细胞膜,青蓝色),细小清蛋白(PV,抑制型神经,紫色),后锚蛋白G(AnkG,神经细胞轴突起始段,绿色),核黄(细胞核,蓝色)
拍摄条件
显微镜:FV3000激光扫描共聚焦显微镜
物镜:20x空气镜(UPLSAPO20X)
激光:405 nm (BV421,核黄),445 nm (BV480),488 nm (AF488),561 nm (AF555),640 nm (AF647)
100x硅油物镜实现深度高分辨率成像
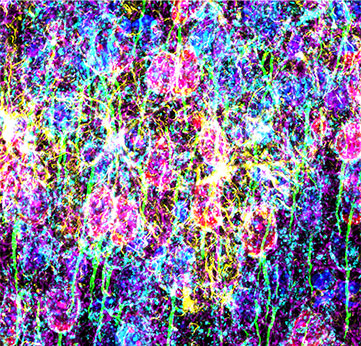
为了获得小鼠内侧前额叶皮层切片高分辨图像,科研人员使用了100x硅油物镜,并配合全真光谱TruSpectral高灵敏检测器,采集到大脑皮层明亮清晰的6色信号。这样的设备组合可以帮助科研人员清晰地捕捉到内侧前额叶皮层不同群体神经元的形态。
(A) | (B) |
图3 小鼠内侧前额叶皮层切片6色标记:神经胶质纤维酸性蛋白(GFAP,小胶质细胞,黄色),钙调蛋白依赖蛋白激酶Ⅱ(CaMKII,锥体细胞,红色),Amphoterin诱导蛋白1前体(AMIGO-1,神经细胞膜,青蓝色),细小清蛋白(PV,抑制型神经,紫色),后锚蛋白G(AnkG,神经细胞轴突起始段,绿色),核黄(细胞核,蓝色)。(A)6色荧光(B)多色叠加图
拍摄条件
显微镜:FV3000激光扫描共聚焦显微镜
物镜:100x硅油镜(UPLSAPO100XS)
激光:405 nm (BV421,核黄),445 nm (BV480),488 nm (AF488),561 nm (AF555),640 nm (AF647)
FV3000共聚焦显微镜如何助力我们的应用?
高灵敏GaAsP检测器和全光谱成像系统高效实现多色荧光成像
| FV3000共聚焦显微镜独有的全真光谱TruSpectral检测技术,与传统的共聚焦光谱检测设备相比具有更高的光通量,得益于体相位全息(VPH) 透射光栅,检测效率比反射光栅高出三倍。同时,FV3000高灵敏光谱检测器(HSD),可以更有效检测微弱荧光信号,每种荧光对应单独检测通道,灵敏度和光谱检测范围独立可调。进一步配合专用的光谱拆分算法,可以提取并拆分光谱序列图像中的重叠光谱信息, 从而解决荧光串色问题。通过实时或离线光谱拆分,最多可实现16色荧光成像。 | Related Videos |
硅油物镜UPLSAPO100XS实现厚标本的明亮成像
UPLSAPO系列超级复消色差物镜在球差和色差方面都有出色的校正,在可见光到近红外范围均有很高的透光率。硅油的折射率(ne≈1.40)接近于活细胞组织的折射率(ne≈1.38),降低了由于介质和活体组织间因折射率不匹配所导致的球差,从而提高了图像分辨率和组织的观察深度。
|
放大倍率:100x
数值孔径NA:1.35(硅油浸物镜) 工作距离WD:0.2mm 色差校准:超级复消色差(SAPO) |
使用FV3000共聚焦显微镜研究神经性疼痛对大脑分子水平的影响
Stephanie Shiers教授的点评:
 | 我们的主要研究方向是神经性疼痛中内侧前额叶皮层依赖的认知功能障碍。通过阐明大脑皮层功能障碍不同细胞类型变化和相应功能的改变,从而进一步研究mPFC的结构可塑性。我们已经观察到疼痛雄性小鼠的在神经认知训练中表现较差可能与mPFC中神经细胞轴突起始段(axon initial segments,AIS)的长度缩小有关(Shiers, et al. J Neurosci 2018, Shiers, et al. Neuropsychopharmacology, 2019)。对于不同神经细胞形态的观察和理解,可以提供更多关于神经性疼痛引起的认知功能障碍的线索,进而提供治疗的思路。FV3000共聚焦多色高分辨率成像对于我们对mPFC微结构的研究非常关键。 |
Theodore Price博士的点评:
 | 我们研究的首要目标是了解认知过程中神经性疼痛如何影响大脑结构变化。认知障碍是神经性疼痛很重要的共发病,但是目前对其分子机理的了解非常少。我们对于小鼠内侧前额叶皮层的研究工作已经发现神经性疼痛会精细地改变神经细胞轴突起始段的结构。这个发现离不开FV3000共聚焦显微镜对于内侧前额叶皮层超微结构高质量成像,无疑为我们对神经性疼痛的研究带来很关键的视角。 |
致谢
感谢以下研究人员协助制作本应用材料
Stephanie Shiers, Ph.D. Candidate, Price Lab, University of Texas at Dallas
Theodore J. Price, Ph.D., Price Lab, Eugene McDermott Professor, Director,
Center for Advanced Pain Studies, Department of Neurobiology, School of Behavioral and Brain Sciences, University of Texas at Dallas
适于这类应用的产品
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
对不起,此内容在您的国家不适用。