From Robert Hooke’s first observation of cube-shaped partitioned cells under his homemade lens, to Osamu Shimomura’s research of jellyfish aequorin and GFP, through to Xiaowei Zhuang’s novel blinking dye molecules during 3D super-resolution reconstruction, we have never stopped exploring the microcosms of life with optical microscopy.
As a mainstay of the fourth industrial revolution, nanotechnology engineering has flourished in the 21st century, initially in materials science applications. When nanotechnology collided with the life sciences, it produced a new spark—fluorescent nanoprobes—brightening up the microcosm of life with the light of nanomaterials.
The Dawn of Fluorescence Microscopy
Fluorescence is a type of photoluminescence and is caused by the absorption of light. There are numerous fluorescent phenomena in nature. The green fluorescent protein (GFP) extracted from luminescent jellyfish was the first practical application of this phenomenon to explore the living microscopic world. In the early 90s, researchers revealed that introducing the GFP gene into other living organisms to express would cause organisms that do not naturally emit fluorescence to produce green fluorescence. Since then, scientists have further improved the available colors, light intensity, stability, and other properties of GFP by analyzing its structure and mechanisms, which greatly advanced the development and application of fluorescent proteins (FPs) in bioimaging (Figure 1).
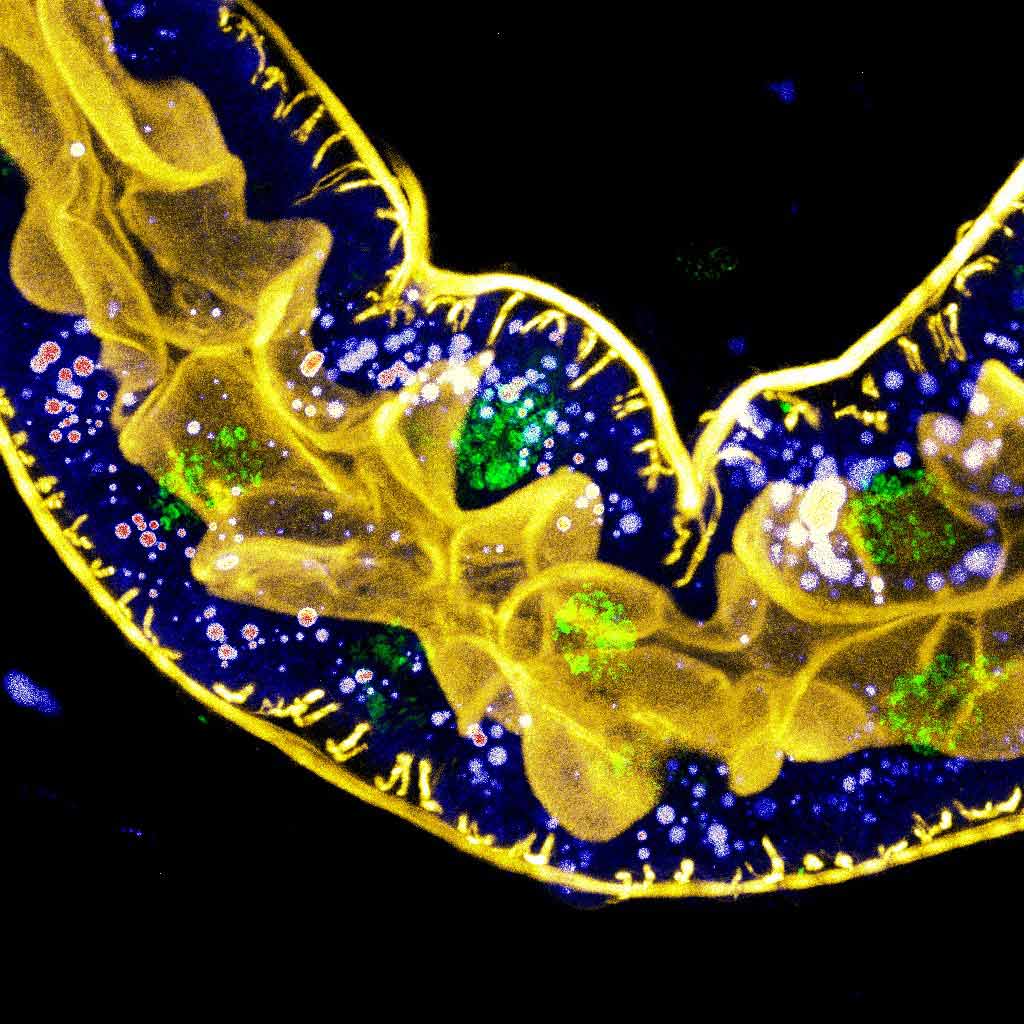
Figure 1. Multicolor fluorescence imaging of a Drosophila larval hindgut where yellow marks f-actin, green marks the nuclei, and intensitometric lookup table (LUT) marks lipid droplets
Although the family of fluorescent proteins is increasingly growing, its application scope is still limited. The emission spectrum of FPs is broad and asymmetric, resulting in frequent cross talk in simultaneous multichannel imaging. With the help of chemical synthesis, organic dyes with rich variety and simple operation emerged, not only greatly expanding the application scope of fluorescent imaging, but also facilitating standard commercial production, which laid a solid foundation for a leap in development.
Nanomaterials Spark Advances in Bioimaging
Gradually, certain disadvantages of organic dyes, such as low fluorescence efficiency and poor photostability, became stumbling blocks to the advancement of fluorescence imaging. Fortunately, owing to inherent special optical properties, directional syntheses and assembly as well as other advantages, nanomaterials are pumping new blood into fluorescence imaging. At present, common fluorescent nanomaterials include the following (Figure 2):
- Semiconductor quantum dots (QDs)
- Upconversion rare earth materials (upconversion nanoparticles, UCNPs)
- Noble metal nanoparticles
Compared with other dyes, fluorescent nanomaterials provide the advantages of high quantum yield, high stability, large Stokes shift, broad excitation spectrum, and narrow emission spectrum. Their emission wavelengths can be changed by size adjustment. Their biocompatibility and recognition and sensing functions can be improved through assembly and modifications. All told, nanomaterials offer excellent potential for fluorescent labeling as well as the future of fluorescence imaging.
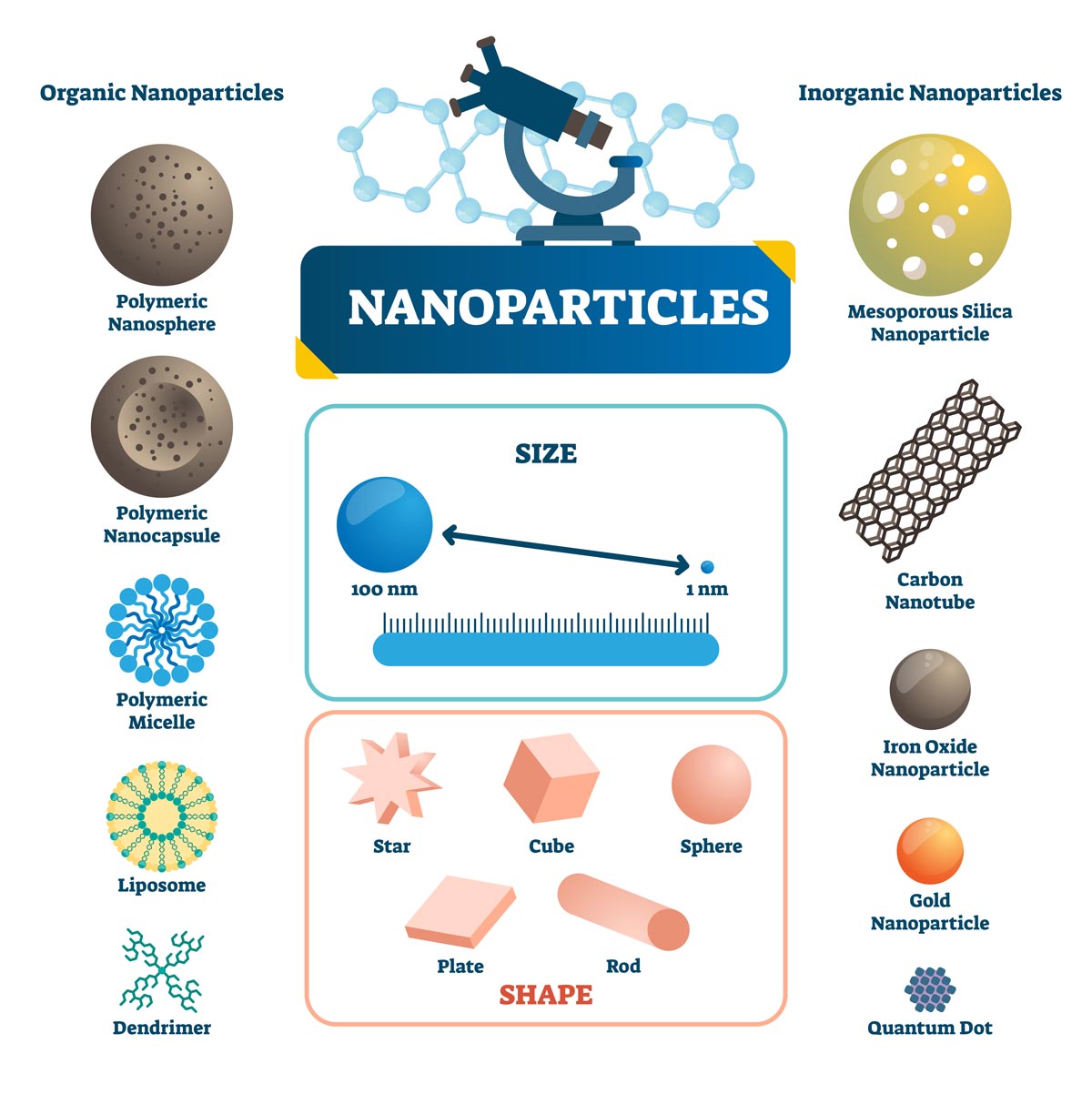
Figure 2. Illustrated examples of organic and inorganic nanoparticles, depicting their size, shapes, and materials
Basic Workflow of Fluorescent Nanoprobes
As it is an interdisciplinary process involving nanochemistry and bioimaging, the workflow for engineering fluorescent nanoprobes is different from that of traditional bioimaging. It is a multistep process including theoretical computation, chemical synthesis, bioimaging, and even medical testing (Figure 3).
Figure 3. Basic workflow of fluorescent nanoprobes
The research processes using fluorescent nanoprobes are mostly application oriented. Bioimaging with fluorescent nanoprobes spans from in vitro samples to in vivo dynamic imaging and can even advance into medical diagnostics and studies. Therefore, the biological application is considered when designing the nanoprobe, and nanomaterials with special optical properties or other functions (for example, near-infrared, upconversion, two-photon, etc.) are selected according to the specific needs of biological applications, and then they are synthesized and characterized to screen for high-quality nanomaterials. Subsequently, the probes are further modified and assembled (Figure 4) by considering their biocompatibility or the needs for their functions, such as optical manipulation, drug loading, molecular recognition, etc.
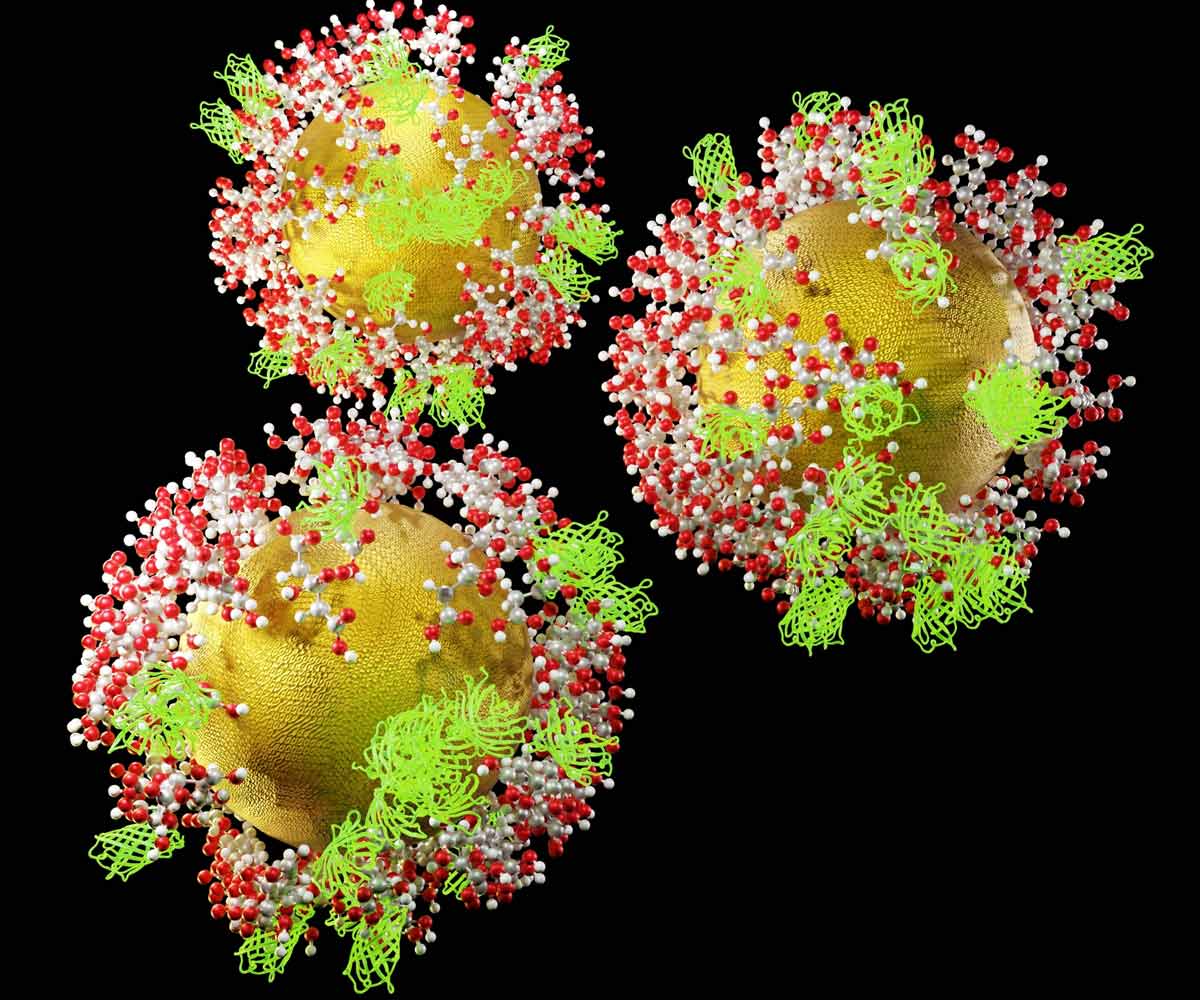
Figure 4. 3D rendering of functionalized gold nanoprobes (GNPs) coated with green fluorescent proteins (GFP) and citric acid molecules
In research involving fluorescent nanoprobes, optical microscopy is mainly applied in three areas: characterization, screening the high-quality nanomaterials, and in vivo detection of nanoprobes. In the screening process, characteristics of different particles are often highly variable because of their inherent structural heterogeneity. In contrast to traditional methods that rely on the ensemble characteristics of a large number of particles together, optical microscopy enables the screening
of high-quality probes at the single-particle level and further investigation of their structure-function relationships to guide their design synthesis. In bioimaging, optical microscopy enables the detection of optical signals of nanoprobes in vivo to image and track their dynamic spatiotemporal reactions.
Research Applications of Fluorescent Nanoprobes and Microscopy Solutions
Near-Infrared/Upconversion Imaging Using a Confocal Microscope Solution
Near-infrared imaging is currently a research hotspot in optical microscopy thanks to its capacity for deep penetration, low phototoxicity, and low interference from tissue autofluorescence. Fluorescent nanoprobes, such as quantum dots, can easily achieve NIR excitation through adjustments to their chemical composition, morphology, and size. In addition, upconversion nanomaterials have a special optical property that is practical for NIR imaging. They emit a shorter wavelength light (visible or UV light) than the excitation light when being excited by a long wavelength light (NIR). Owing to these advantages, upconversion nanoprobes are considered as new-generation fluorescent biomarkers, and they are expected to play an important role in biomedicine, energy and catalysis research, and other fields.
Sensitive and real-time in vivo detection of hepatotoxicity is currently a bottleneck in the diagnosis of drug-induced liver injury. In a paper published in 2020 by Professor Li Huijun's research group, an upconverting nanoprobe was designed by assembling upconversion nanoparticles (UCNPs) and gold nanorods (GNR) and was applied to in situ real-time diagnosis of drug-induced liver injury. This novel nanoprobe can aggregate in the liver and be specifically activated by a liver injury marker, miR122, producing fluorescence images at 800 nm when being excited by near-infrared light at 980 nm. In combination with luminescence resonance energy transfer (LRET) and signal amplification technology, its detection sensitivity is further increased to achieve highly sensitive detection of miR122, providing a new approach for real-time clinical monitoring of drug-induced liver injury.[4]
In contrast to conventional confocal imaging, imaging of NIR/upconverting nanoprobes requires equipment with certain capabilities:
- Commonly used excitation wavelengths of conventional confocal microscopy are 400–650 nm, whereas near-infrared imaging requires NIR lasers using wavelengths >700 nm
- Most optical path components used in conventional imaging, such as scanning galvanometer, objectives, and diffraction gratings, only support antireflection/calibration in the visible range, and cannot provide NIR’s imaging efficiency and accuracy
- NIR detection requires dedicated >750 nm NIR detectors
To satisfy these requirements, the FLUOVIEW™ FV4000 laser confocal microscope offers an NIR imaging solution (Figure 5). The FV4000 system specializes in highly sensitive and accurate NIR imaging with more colors.
|  |
Figure 5. The modular FLUOVIEW FV4000 confocal laser scanning system offers an NIR imaging solution |
In this application note prepared in collaboration with Dr. Kai Li (Southern University of Science and Technology, China), you can read about the successful use of the FLUOVIEW NIR solution to determine the targeting ability of NIR fluorescence molecule, BTB based nanoparticles on tumor cells. (Fig6) The researchers in this study modified the BTB nanoprobe’s surface with the Arg-Gly-Asp (RGD) targeting peptide and observed that over 48 hours the mouse injected with BTB-RGD NPs showed a significant increase in NIR-II fluorescence signal at the tumor site compared with the BTB nanoprobe.
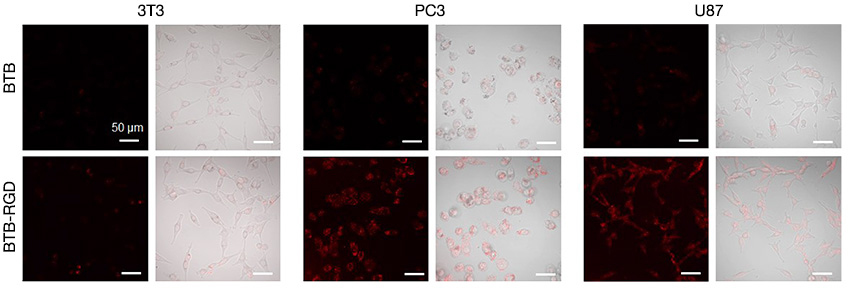
Figure 6. Confocal images of 3T3, PC3, and U87 cells by FV3000 Red after incubation with 40 μg mL-1 of BTB-RGD or BTB NPs for 4 hours.
Excitation wavelength: 730 nm, filter: 760–890 nm, scale bar = 50 μm.
In Vivo Imaging of Tumor Markers Using the FLUOVIEW Multiphoton Microscope Deep-Detection Solution
Cancer is one of society’s most serious public health concerns. Early diagnosis and accurate excision of malignant tumors is a top priority of current medical research. The design and development of novel fluorescent nanoprobes for specific identification of tumor markers and high-resolution in vivo imaging, can provide new detection solutions for early clinical diagnosis of malignant tumors as well as accurate surgical excision.
Over expression of enzymes, such as alkaline phosphatase (ALP), in tumor cells is an important clinical indicator of occurrence, development, and progression of tumors. Therefore, a rapid detection method that offers high sensitivity to ALP activity can help in early detection and accurate excision of tumors. In 2020, Professor Xiaojun Peng and his team published a paper on the use of a nanoprobe designed for just such a purpose. The paper detailed how they designed and prepared an AIEgen probe (DQM-ALP) that emits strong fluorescence upon aggregation after interacting with over-expressed ALPs in tumor cells. This engineered nanoprobe avoided the fluorescence quenching problem caused by aggregation in conventional organic dyes, thereby increasing the detection sensitivity and retention time of the probe in tumor cells. This work was the first to discover the up-regulation of ALP activity in tumor cells by sodium butyrate and cortisol stimulation, and they used two-photon microscopy to achieve the high spatial resolution three-dimensional deep imaging of ALP activity in HeLa and HepG-2 tumor spheroids. The paper demonstrated the usefulness of the probe in fluorescence detection imaging of submillimeter tumors, providing a powerful support tool for clinical diagnosis and surgical excision of tumors.[5]
One- and two-photon confocal microscopes were used in the above study. However, the latest FLUOVIEW multiphoton microscope, which is designed for in vivo deep fluorescence imaging with a proprietary high-speed resonant scanning galvanometer and a highly sensitive optical detection path, can achieve high-resolution 3D deep imaging of tumor markers in a more accurate and efficient manner (Figure 7).
The combination of dual-line/dual lasers and high-sensitivity detection on up to six channels in Evident’s multiphoton system supports more flexible multicolor imaging, further increasing detection throughput and efficiency.
|  |
Figure 7. The FV4000MPE multiphoton system with modules for deep imaging |
Dynamic Detection of Nanoprobes in Live Cells Using the IXplore™ SpinSR Rapid Super Resolution Solution
Biochemical reactions and other molecular events in living cells often have significant spatiotemporal dynamic characteristics. Optical imaging technology can precisely track the motion of nanoprobes and study their interactions with biomolecules. It enables researchers to effectively monitor these biomarkers to observe dynamic changes for further exploration of the relationships between their states and relevant cellular functions. Endosomes, lysosomes, and other organelles play key roles in signal transduction and maintenance of metabolic homeostasis. Since pHs of these organelles will change during endocytosis, rapid and sensitive detection of pH values of these organelles has become the focus of research on dynamical monitoring of endocytosis process and the exploration of the relationships between their states and cellular functions.
In 2016, Professor Yiguang Wang from Peking University and Professor Jinming Gao’s research team from the Southwestern Medical Center of the University of Texas published a paper in which they designed an ultra-sensitive pH nanoprobe (HyUPS) at single-organelle resolution. They used this nanoprobe to track and detect pH changes in endosomes/lysosomes and other organelles during endocytosis. The probe includes three pH sensitive components corresponding to the three components of endocytosis and three pH ranges, and are labeled with red, green, and blue fluorophores respectively. The probe successfully achieved real-time and multicolor dynamic monitoring of acidification rate in organelles involved in endocytosis in living cells, providing a new imaging tool for further studies of endosomal/lysosomal dysfunctional diseases.[6]
Nanoprobe imaging in a live specimen requires high-speed imaging equipment, and the conventional point-scanning confocal approach fails to meet the requirements. The IXplore SpinSR spinning disk confocal super resolution imaging system, can achieve fast (200 fps) multicolor imaging at super resolution (110 nm) quality (Figure 8). These capabilities enable it to capture the fast dynamic processes of fine structures, making it a powerful tool for life science researchers to easily improve their imaging efficiency. The proprietary RTCe (real-time controller) controls the synchronized operation of all components to minimize the impact of excitation light on samples. This advantage, in combination with silicone oil objectives designed for deep biopsy, enables long-term, accurate deep imaging of live cells, spheroids, and organoid samples.
See an example of the super resolution imaging capabilities of the IXplore SpinSR system in Figure 9, showing epithelial cell mitochondria stained with PKMDR dye. Developed by Professor Zhixing Chen from Peking University, PKMDR is a mitochondrial probe that minimizes phototoxicity in fluorescence and nanoscopic imaging.
IXplore SpinSR spinning disk confocal system
| |
Figure 8. The IXplore SpinSR spinning disk confocal super resolution imaging system |
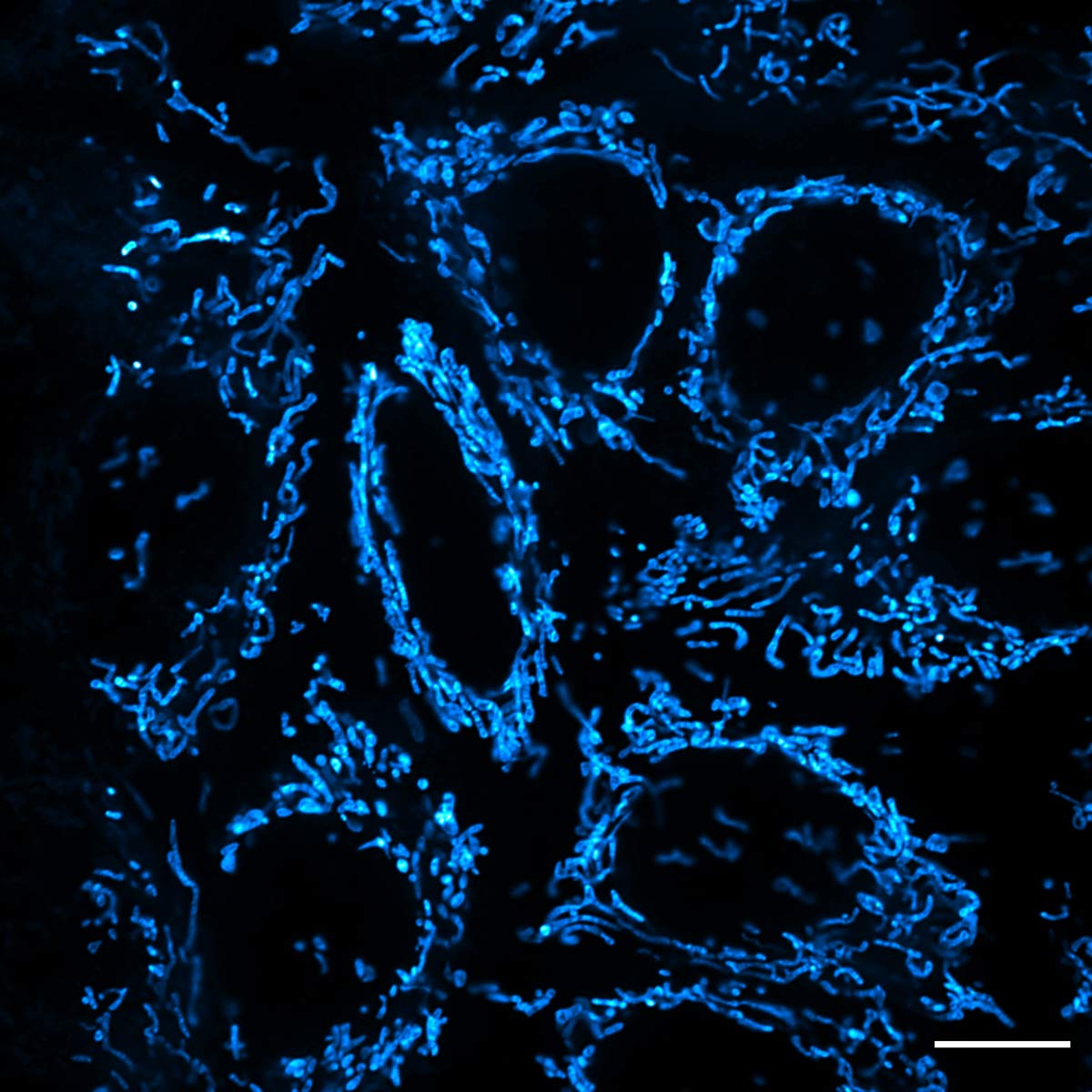
Figure 9. Super resolution image of epithelial cell mitochondria stained with PKMDR dye using the IXplore SpinSR system, UPLAPO60XOHR objective, and TruSight™ processing. Image courtesy: Dr. Huiwen Hao, Dr. Junsheng Yang, Prof. Yujie Sun, and Prof. Zhixing Chen. Standard Imaging Co., Ltd. and Sun Lab, College of Future Technology, PKU. NanJing GenVivo Biotech Co., Ltd.
In the past decade, with the rapid development of nanotechnology and optical imaging technologies, many important advances have been made in biochemical imaging applications using fluorescent nanoprobes. However, the application of fluorescent nanoprobes is still limited by their biocompatibility, presence of fluorescence blinking behavior, and other shortcomings. With the continuous development and integration of nanomaterials and bioimaging technologies, chemical synthesis, theoretical physics, and image analysis, it is expected that the specificity, accuracy, stability, and reproducibility of fluorescent nanoprobe bioimaging will be further improved, expanding its potential to play a more important role in wider range of research fields.
*This article was updated based on original content published on October 6, 2022.
References:
[2]. Chang, H., Xie, J., Zhao, B., Liu, B., Xu, S., Ren, N., Xie, X., Huang, L., & Huang, W. (2014). Rare Earth Ion-Doped Upconversion Nanocrystals: Synthesis and Surface Modification. Nanomaterials (Basel, Switzerland), 5(1), 1–25.
[3]. Chen, Z., Wu, X., Hu, S., Hu, P., & Liu, Y. Multicolor upconversion NaLuF4 fluorescent nanoprobe for plant cell imaging and detection of sodium fluorescein. J. Mater. Chem. C, 2015, 3, 153-161
Related Contents
Enhanced Darkfield Illumination: Label-Free Imaging at the Nanoscale
On the Road to a Breakthrough—Trailblazing Bioimaging Is Transforming Cancer Research

.jpg?rev=1A74)